Question: I need help completing the table below regarding the concentrations of mixtures. For reference, I have included the lab instructions below the table I need
I need help completing the table below regarding the concentrations of mixtures. For reference, I have included the lab instructions below the table I need assistance with. Thank you in advance!
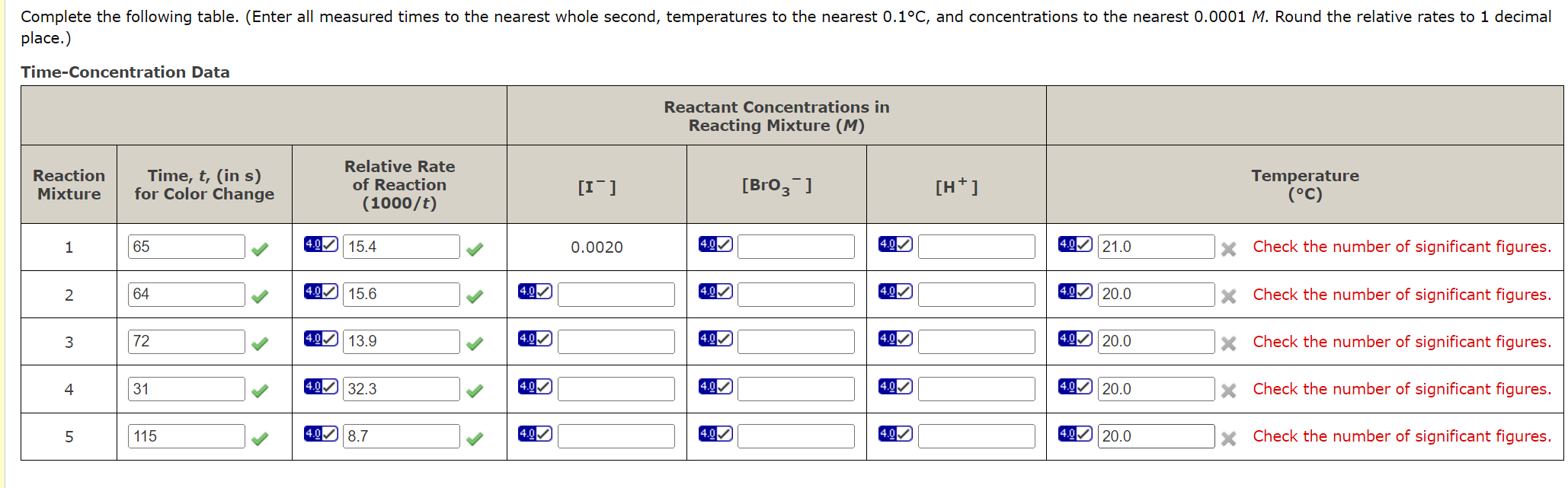
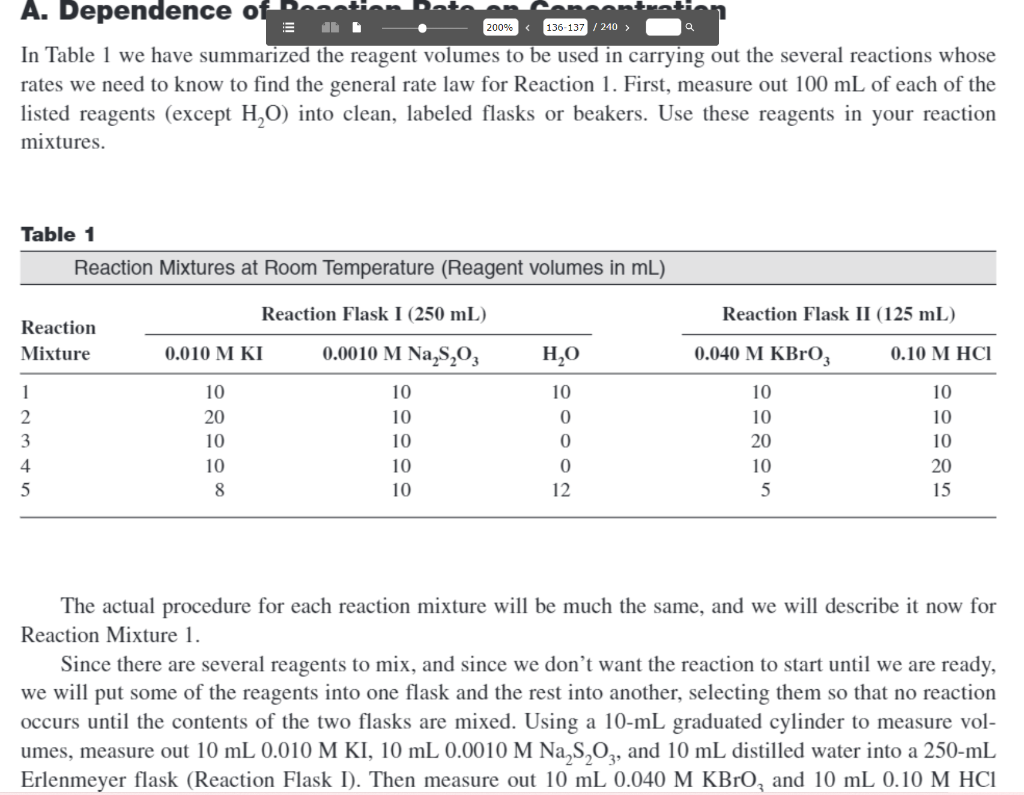
place.) Time-Concentration Data In Table 1 we have summarized the reagent volumes to be used in carrying out the several reactions whose rates we need to know to find the general rate law for Reaction 1. First, measure out 100mL of each of the listed reagents (except H2O ) into clean, labeled flasks or beakers. Use these reagents in your reaction mixtures. Table 1 The actual procedure for each reaction mixture will be much the same, and we will describe it now for Reaction Mixture 1. Since there are several reagents to mix, and since we don't want the reaction to start until we are ready, we will put some of the reagents into one flask and the rest into another, selecting them so that no reaction occurs until the contents of the two flasks are mixed. Using a 10-mL graduated cylinder to measure volumes, measure out 10mL0.010MKI,10mL0.0010MNa2S2O3, and 10mL distilled water into a 250-mL Erlenmeyer flask (Reaction Flask I). Then measure out 10mL0.040MKBrO3 and 10mL0.10MHCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


