Question: I need help converting the absorbance rates to moles/ second using Beer's law. In question 2. Then need help in question 3 please. I gave

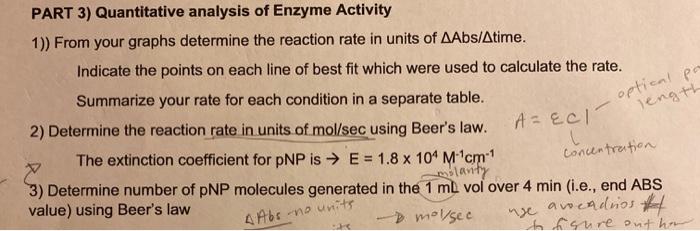 I need help converting the absorbance rates to moles/ second using Beer's law. In question 2. Then need help in question 3 please. I gave the absorbance rates in the 2nd picture, the other charts per experiment are for reference.
I need help converting the absorbance rates to moles/ second using Beer's law. In question 2. Then need help in question 3 please. I gave the absorbance rates in the 2nd picture, the other charts per experiment are for reference.
Experiment I Experiment 2 Experiment 3 PH 6.5, 37C 2X- HP kate = 0.001 2x- PNPP %3D Rorte = 0.0L Rate = D.00595 sptimam pH 6.5,70'C Rate = 0,0005 IX-PNPP Rate = 0.007 Rate = 0.0o53 PH 6.5, 100C Y2 x- PNPP Rate = 0. 00006 kate = 0.005 Rate= 0.00 366 PH 10.4, 37C Vu x- PN Pe Rate = 0.005 optimum Rate = 0.002 Rate= 0.0o7 pH 10.4,77C Rate z D001 DX- PNPP Rate = 0.001 Rate= 0.000I pH lo,4, lo0c Rate = 0.001b
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
To solve this we need to apply Beers Law and some basic calculations Heres a stepbystep guide Understanding Beers Law Beers Law is given by A varepsil... View full answer

Get step-by-step solutions from verified subject matter experts


