Question: i need help doing this problem! this is second 2 which you use for question 3 Problem 3 Draw a diagram with energy on the
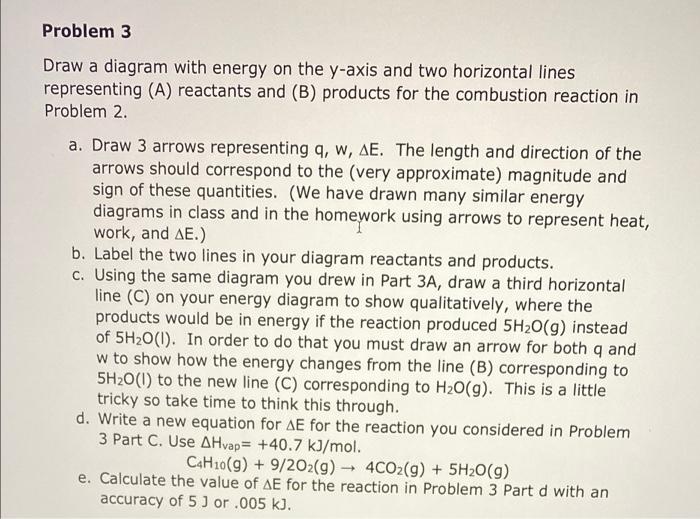

Problem 3 Draw a diagram with energy on the y-axis and two horizontal lines representing (A) reactants and (B) products for the combustion reaction in Problem 2. a. Draw 3 arrows representing q, w, AE. The length and direction of the arrows should correspond to the (very approximate) magnitude and sign of these quantities. (We have drawn many similar energy diagrams in class and in the homework using arrows to represent heat, work, and AE.) b. Label the two lines in your diagram reactants and products. C. Using the same diagram you drew in Part 3A, draw a third horizontal line (C) on your energy diagram to show qualitatively, where the products would be in energy if the reaction produced 5H20(9) instead of 5H20(1). In order to do that you must draw an arrow for both q and w to show how the energy changes from the line (B) corresponding to 5H20(1) to the new line (C) corresponding to H2O(9). This is a little tricky so take time to think this through. d. Write a new equation for AE for the reaction you considered in Problem 3 Part C. Use A Hvap= +40.7 kJ/mol. C4H10(g) + 9/202(g) + 4CO2(g) + 5H20 (9) e. Calculate the value of AE for the reaction in Problem 3 Part d with an accuracy of 5 ) or .005 k]. - Problem 2 - 2. Consider this combustion reaction. C4H10(9) + 9/202(9) 4CO2(g) + 5H20(1) AH = -2959.4 kJ/mol a. Write the full equation for AE when 16 g of CO2 are formed at 1 atm and 340. K. I used 44.01 for the molar mass of CO2. b. Calculate the value of AE with an accuracy of 5 ) or .005 k). C. There are 4 key terms that should be in your equation. Remember to include units. Identify what each term in the equation means and give an explanation stating why the term is needed or what its purpose is. A "term" is a group of symbols such as 16.0 g/44.01 g/mol. For your combustion problem make sure you identify (A) Problem 3 Draw a diagram with energy on the y-axis and two horizontal lines representing (A) reactants and (B) products for the combustion reaction in Problem 2. a. Draw 3 arrows representing q, w, AE. The length and direction of the arrows should correspond to the (very approximate) magnitude and sign of these quantities. (We have drawn many similar energy diagrams in class and in the homework using arrows to represent heat, work, and AE.) b. Label the two lines in your diagram reactants and products. C. Using the same diagram you drew in Part 3A, draw a third horizontal line (C) on your energy diagram to show qualitatively, where the products would be in energy if the reaction produced 5H20(9) instead of 5H20(1). In order to do that you must draw an arrow for both q and w to show how the energy changes from the line (B) corresponding to 5H20(1) to the new line (C) corresponding to H2O(9). This is a little tricky so take time to think this through. d. Write a new equation for AE for the reaction you considered in Problem 3 Part C. Use A Hvap= +40.7 kJ/mol. C4H10(g) + 9/202(g) + 4CO2(g) + 5H20 (9) e. Calculate the value of AE for the reaction in Problem 3 Part d with an accuracy of 5 ) or .005 k]. - Problem 2 - 2. Consider this combustion reaction. C4H10(9) + 9/202(9) 4CO2(g) + 5H20(1) AH = -2959.4 kJ/mol a. Write the full equation for AE when 16 g of CO2 are formed at 1 atm and 340. K. I used 44.01 for the molar mass of CO2. b. Calculate the value of AE with an accuracy of 5 ) or .005 k). C. There are 4 key terms that should be in your equation. Remember to include units. Identify what each term in the equation means and give an explanation stating why the term is needed or what its purpose is. A "term" is a group of symbols such as 16.0 g/44.01 g/mol. For your combustion problem make sure you identify (A)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


