Question: I need help filing in this table and calculating pH and buffer capacity Chemicals & Reagents pH 4.01 and pH 7.00 Standard solutions 0.1 M
I need help filing in this table and calculating pH and buffer capacity
Chemicals & Reagents pH 4.01 and pH 7.00 Standard solutions 0.1 M Na2HPO4 (sodium phosphate dibasic) 0.1 M KH2PO4 (potassium phosphate monobasic) 0.1 M NaOH (sodium hydroxide)
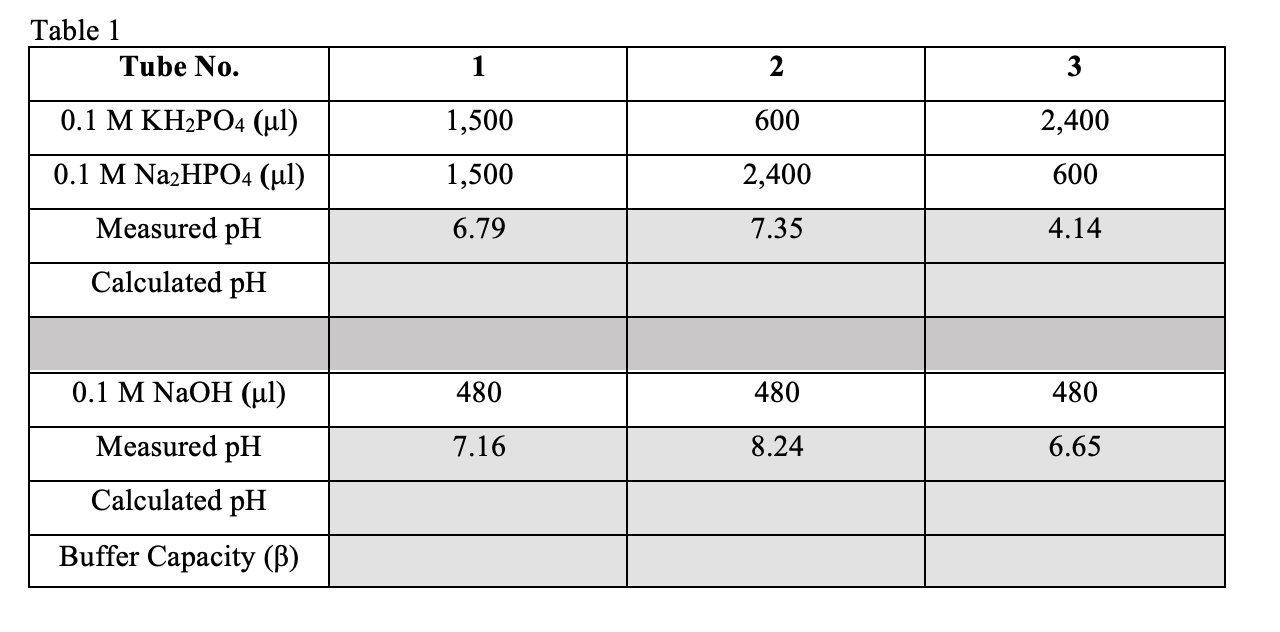
Table 1 \begin{tabular}{|c|c|c|c|} \hline Tube No. & 1 & 2 & 3 \\ \hline 0.1MKH2PO4(l) & 1,500 & 600 & 2,400 \\ \hline 0.1MNa2HPO4(l) & 1,500 & 2,400 & 600 \\ \hline Measured pH & 6.79 & 7.35 & 4.14 \\ \hline Calculated pH & & & \\ \hline & & & 480 \\ \hline 0.1MNaOH(l) & 480 & 8.24 & 6.65 \\ \hline Measured pH & 7.16 & & \\ \hline Calculated pH & & & \\ \hline Buffer Capacity () & & & \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


