Question: i need help for the three questions Consider the reaction: 2NH3(g)+3N2O(g)4N2(g)+3H2O(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.73 moles

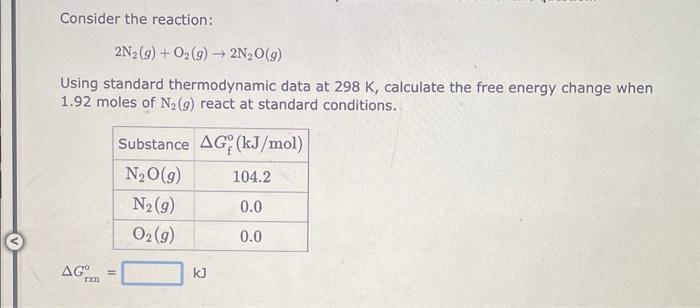
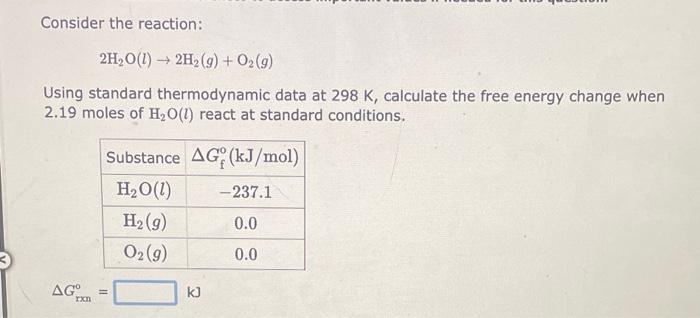
Consider the reaction: 2NH3(g)+3N2O(g)4N2(g)+3H2O(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.73 moles of NH3(g) react at standard conditions. Gixn=kJ Consider the reaction: 2N2(g)+O2(g)2N2O(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.92 moles of N2(g) react at standard conditions. Consider the reaction: 2H2O(l)2H2(g)+O2(g) Using standard thermodynamic data at 298K, calculate the free energy change when 2.19 moles of H2O(l) react at standard conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


