Question: i need help for the three questions For the reaction CH4(g)+H2O(g)3H2(g)+CO(g) H=206kJ and S=215J/K The equilibrium constant for this reaction at 255.0K is Assume that
i need help for the three questions 
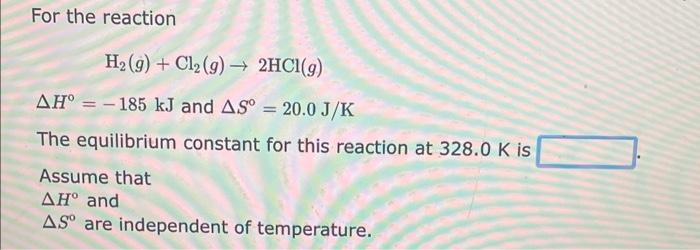
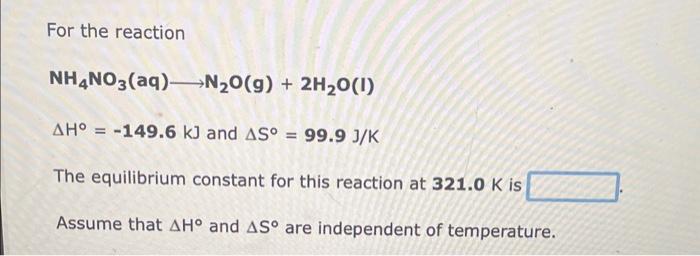

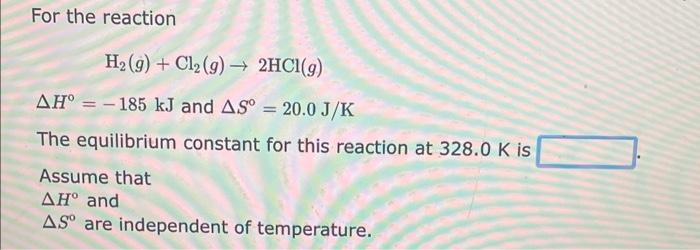

For the reaction CH4(g)+H2O(g)3H2(g)+CO(g) H=206kJ and S=215J/K The equilibrium constant for this reaction at 255.0K is Assume that H and S are independent of temperature. For the reaction H2(g)+Cl2(g)2HCl(g) H=185kJandS=20.0J/K The equilibrium constant for this reaction at 328.0K is Assume that H and S are independent of temperature. For the reaction NH4NO3(aq)N2O(g)+2H2O(I)H=149.6kJandS=99.9J/K The equilibrium constant for this reaction at 321.0K is Assume that H and S are independent of temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


