Question: i need help for the three questions Predict whether S for each reaction would be greater than zero, less than zero, or too close to
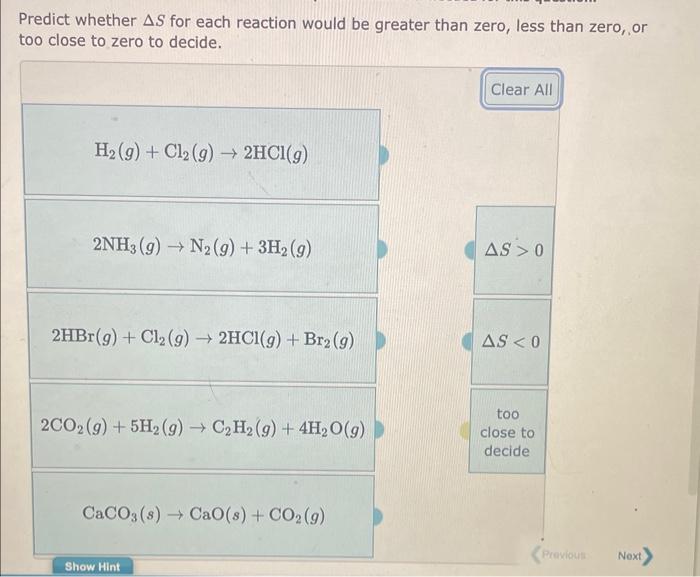


Predict whether S for each reaction would be greater than zero, less than zero, or too close to zero to decide. H2(g)+Cl2(g)2HCl(g) 2HBr(g)+Cl2(g)2HCl(g)+Br2(g) S0. Choose all that apply. S(s,rhombic)+2CO(g)SO2(g)+2C(s,graphite)2C2H6(g)+7O2(g)4CO2(g)+6H2O(g)CH4(g)+H2O(g)CO(g)+3H2(g)2H2(g)+O2(g)2H2O(g)NH4HS(s)NH3(g)+H2S(g) Which of the following transformations represent an increase in the entropy of the system. Choose all that apply 42gC2H5OH(liquid,167K)42gC2H5OH(liquid,344K)40gAl(liquid,933.0K)40gAl(solid,933.0K)2molO2(1.90atm,477K)2molO2(1.90atm,238K)42gC2H5OH(liquid,352K)42gC2H5OH(gas,352K)1molCH4(7.96L,240K)1molCH4(15.9L,240K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


