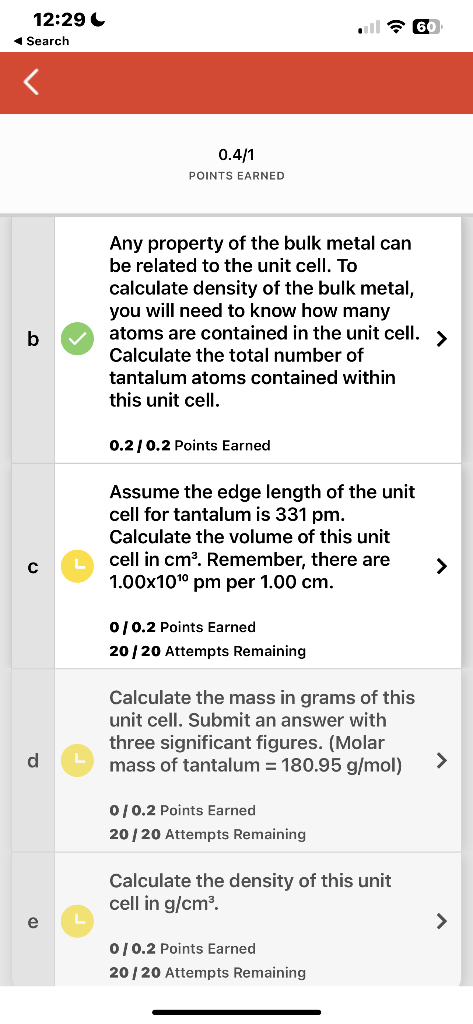
Question: I need help on this 0.4/1 POINTS EARNED Any property of the bulk metal can be related to the unit cell. To calculate density of
 I need help on this
I need help on this
0.4/1 POINTS EARNED Any property of the bulk metal can be related to the unit cell. To calculate density of the bulk metal, you will need to know how many atoms are contained in the unit cell. > Calculate the total number of tantalum atoms contained within this unit cell. 0.2/0.2 Points Earned Assume the edge length of the unit cell for tantalum is 331pm. Calculate the volume of this unit cell in cm3. Remember, there are 1.001010pm per 1.00cm. 0/0.2 Points Earned 20/20 Attempts Remaining Calculate the mass in grams of this unit cell. Submit an answer with three significant figures. (Molar mass of tantalum =180.95g/mol)> 0/0.2 Points Earned 20/20 Attempts Remaining Calculate the density of this unit cell in g/cm3. 0/0.2 Points Earned 0.4/1 POINTS EARNED Any property of the bulk metal can be related to the unit cell. To calculate density of the bulk metal, you will need to know how many atoms are contained in the unit cell. > Calculate the total number of tantalum atoms contained within this unit cell. 0.2/0.2 Points Earned Assume the edge length of the unit cell for tantalum is 331pm. Calculate the volume of this unit cell in cm3. Remember, there are 1.001010pm per 1.00cm. 0/0.2 Points Earned 20/20 Attempts Remaining Calculate the mass in grams of this unit cell. Submit an answer with three significant figures. (Molar mass of tantalum =180.95g/mol)> 0/0.2 Points Earned 20/20 Attempts Remaining Calculate the density of this unit cell in g/cm3. 0/0.2 Points Earned
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


