Question: I need help on this exercise, if you could explain the steps i would highly appreciate it !!! A mixture of 45 mol% benzene and
I need help on this exercise, if you could explain the steps i would highly appreciate it !!!
A mixture of 45 mol% benzene and 55% toluene is subjected to flash distillation at a pressure in the separator of 1 atm. The incoming liquid is heated to a temperature that will cause 50% of the feed to evaporate. At the feed temperature, the vapor pressure for benzene and toluene is P(benzene) =1.210. mmHg and P(toluene) = 492. mmHg. (Remember AB = PA/PB)
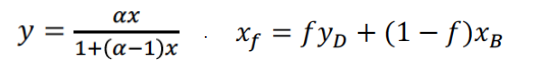
Using the above information, calculate: (Take all answers to two decimal places)
xF =
f =
1-f =
AB =
The coefficients for the quadratic equation in xB, (resulting from solving the two equations substituting YD in the xF equation) are: (Each number must include its own sign)
_________xB2 ________ xB _________= 0
Then solving the resulting quadratic:
xB= _______ yD= _______
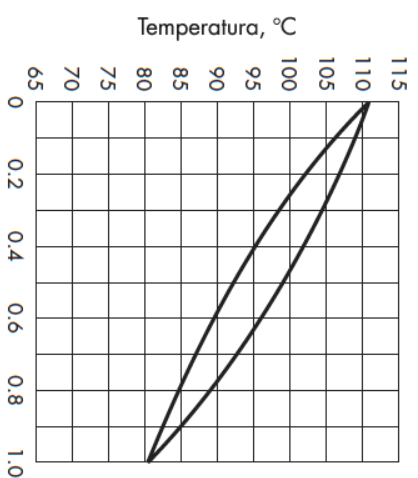
For the resulting values of xB and yD, the temperature corresponding to the equilibrium of that mixture is:
T calc= ________C
y=1+(1)xxxf=fyD+(1f)xB =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


