Question: I need help solving these questions. Gas Equilibrium Problems (First Set) 1) 2HCl(g)H2(g)+Cl2(g) K=1.00102; Initial [HCl]=1.50101M What is [all species] at equilibrium? 2) H2(g)+Br2(g)2HBr(g) K=1.00108;
I need help solving these questions.
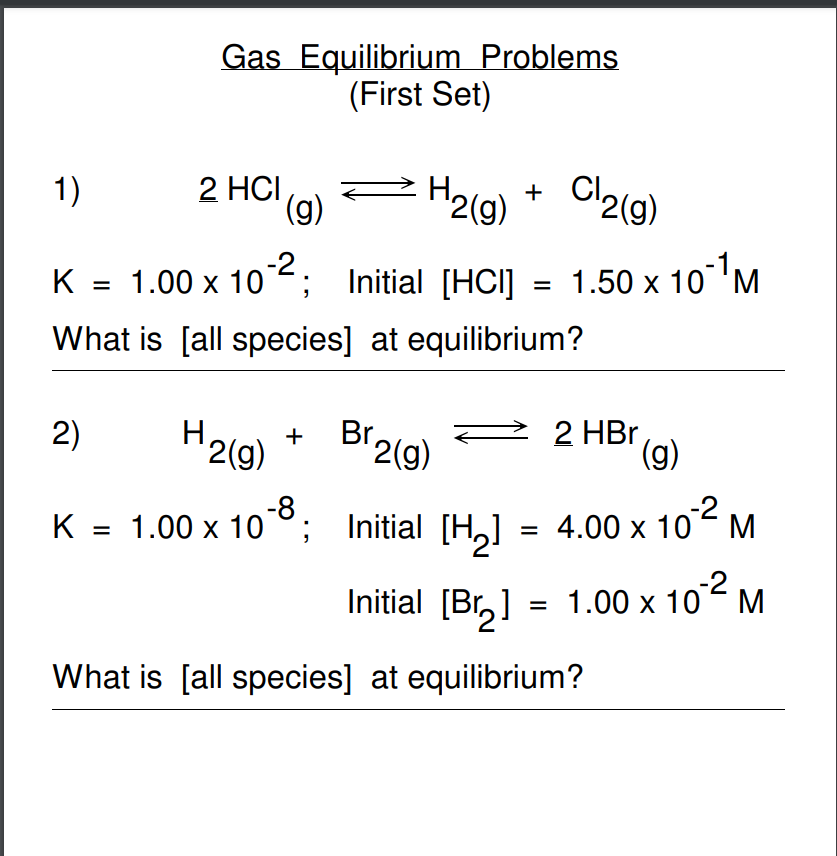
Gas Equilibrium Problems (First Set) 1) 2HCl(g)H2(g)+Cl2(g) K=1.00102; Initial [HCl]=1.50101M What is [all species] at equilibrium? 2) H2(g)+Br2(g)2HBr(g) K=1.00108; Initial [H2]=4.00102M Initial [Br2]=1.00102M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


