Question: I need help solving this. thank you 11.(Bonus-1 point): Consider the reaction: 3H2(g)+N2(g)2NH3(g). If [NH3]=0.78M,[H2]=0.44M, and [N2]=0.88M at equilibrium, what is the value of K
I need help solving this. thank you 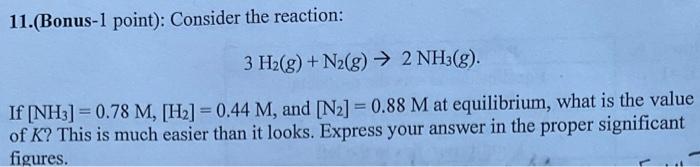
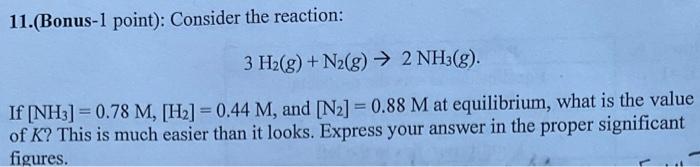
11.(Bonus-1 point): Consider the reaction: 3H2(g)+N2(g)2NH3(g). If [NH3]=0.78M,[H2]=0.44M, and [N2]=0.88M at equilibrium, what is the value of K ? This is much easier than it looks. Express your answer in the proper significant
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


