Question: I need help with C, not sure my answer is correct. I got 0.067 m/s. Please write clearly and show steps! Thanks. Consider the equation:
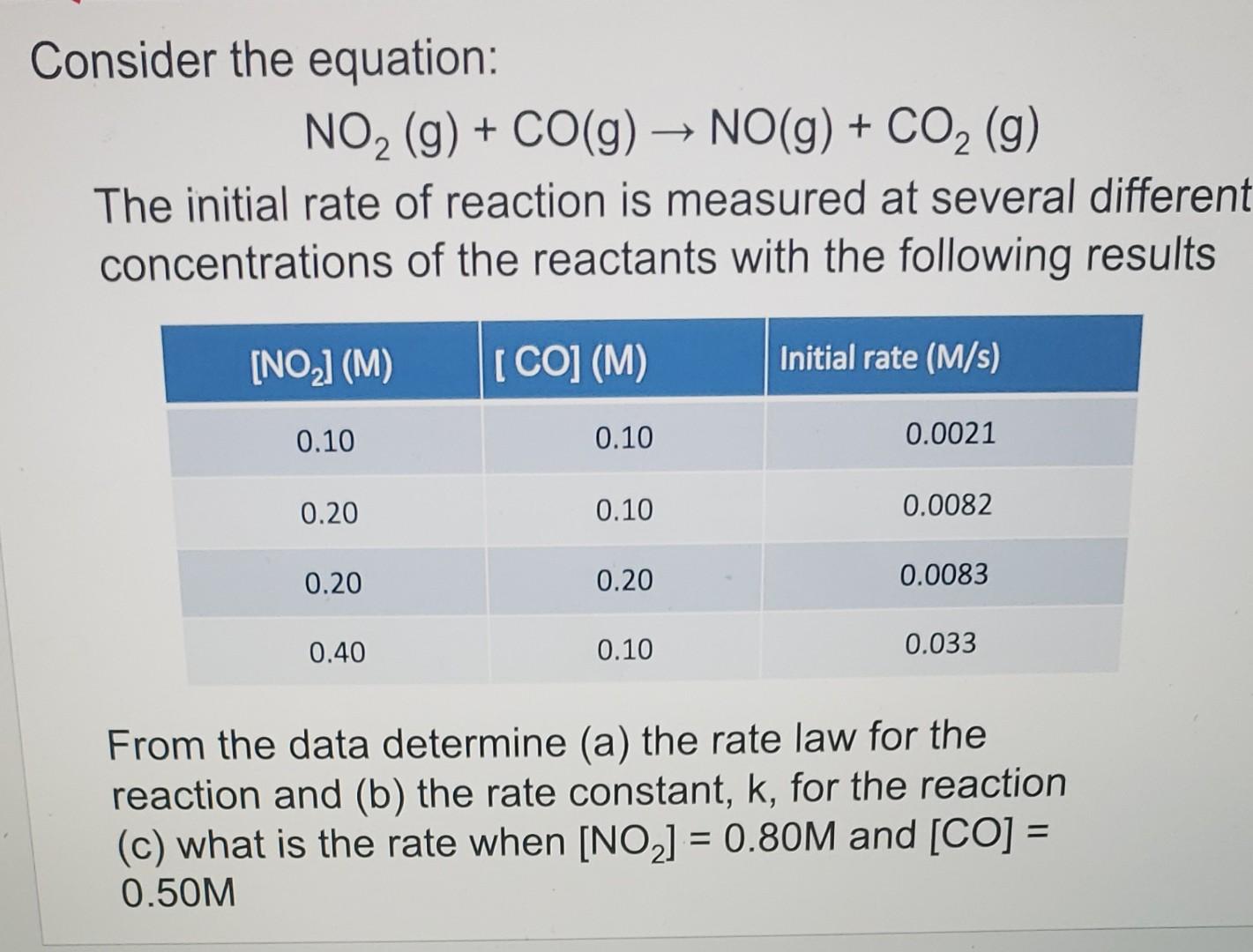
I need help with C, not sure my answer is correct. I got 0.067 m/s. Please write clearly and show steps! Thanks.
Consider the equation: NO2(g)+CO(g)NO(g)+CO2(g) The initial rate of reaction is measured at several differen concentrations of the reactants with the following results From the data determine (a) the rate law for the reaction and (b) the rate constant, k, for the reaction (c) what is the rate when [NO2]=0.80M and [CO]= 0.50M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


