Question: I need help with d D- The cryoscopic constant (Kf) for benzene is 5.12CKgmol1,23.56g of an Unknown molecular solid is dissolved in 500.0g of benzene.

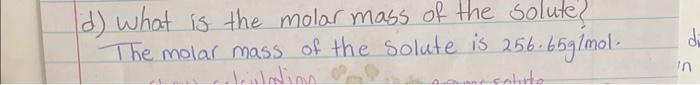
D- The cryoscopic constant (Kf) for benzene is 5.12CKgmol1,23.56g of an Unknown molecular solid is dissolved in 500.0g of benzene. The resulting freezing point of the Solution is 0.40.94C lower than that of pure benzene (i.e, T=0.94C ). calculate the molar mass of the unknown compound. d) What is the molar mass of the solute? The molar mass of the solute is 256.65g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


