Question: i need help with number 2 it says intermediates are correct but curved arrows are incorrect Atten 1,3-Butadiene undergoes an electrophilic addition with ( ce(HBr}
i need help with number 2 it says intermediates are correct but curved arrows are incorrect
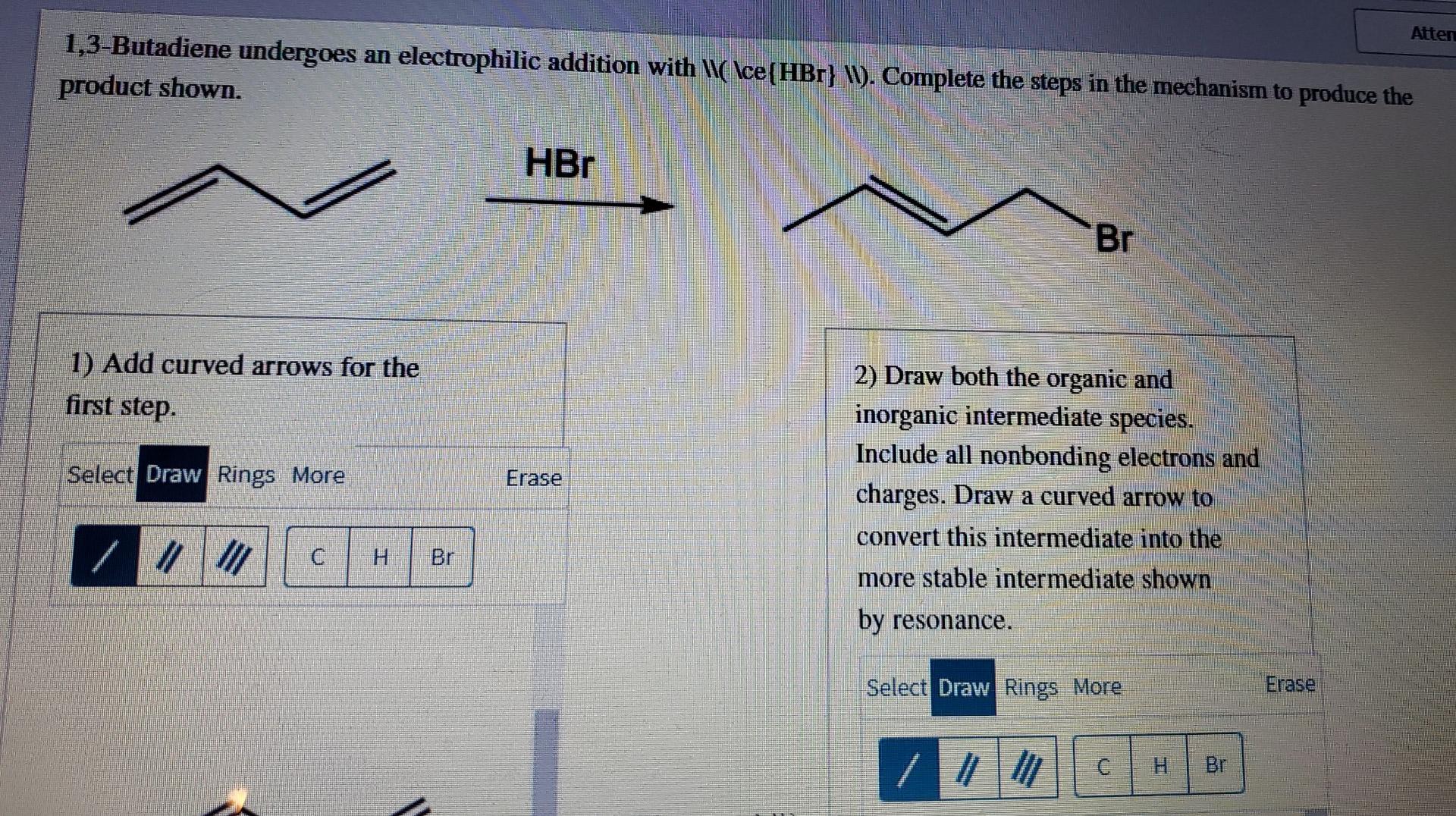
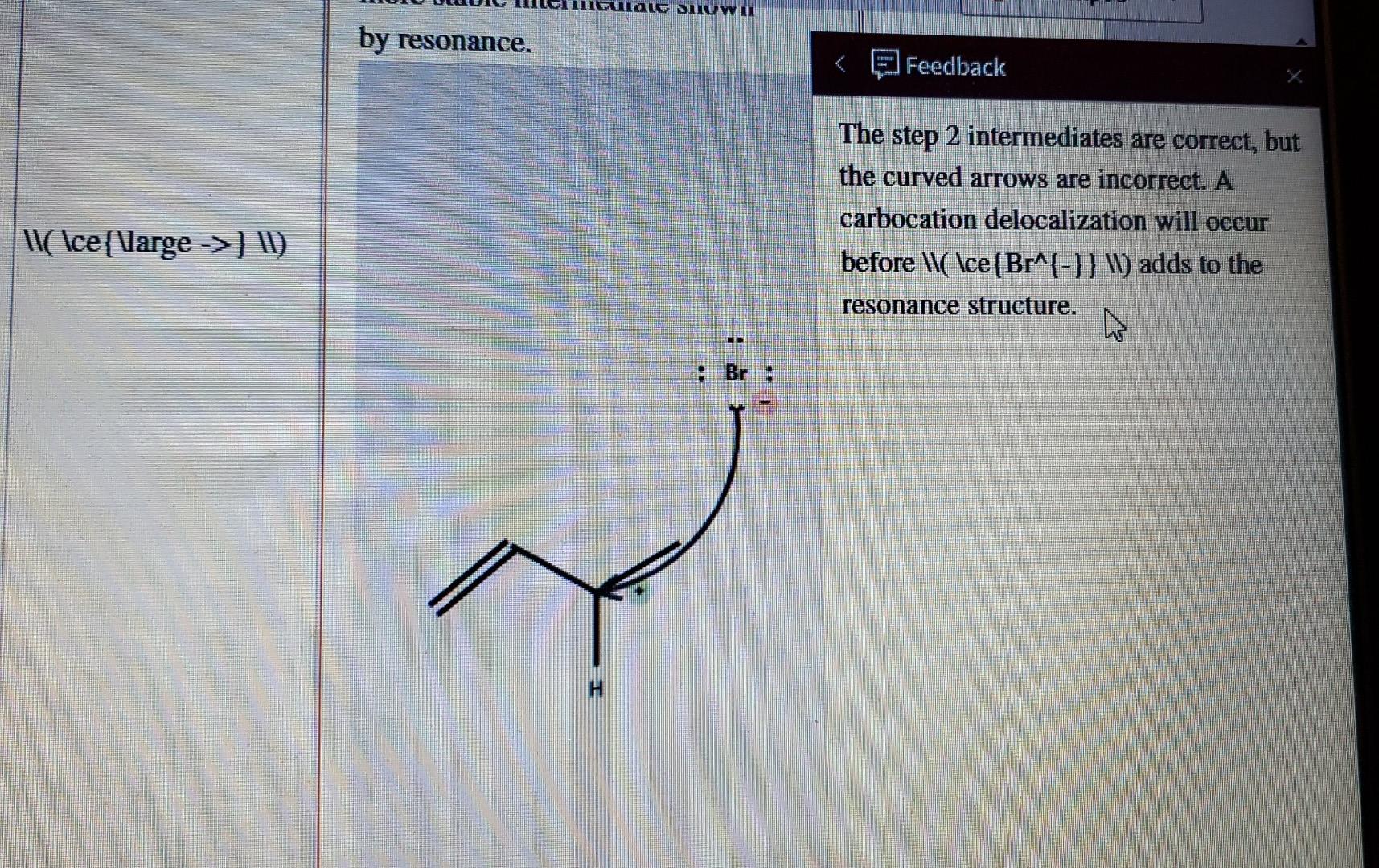
Atten 1,3-Butadiene undergoes an electrophilic addition with \\( \ce(HBr} \). Complete the steps in the mechanism to produce the product shown. HBr Br 1) Add curved arrows for the first step. Select Draw Rings More Erase 2) Draw both the organic and inorganic intermediate species. Include all nonbonding electrons and charges. Draw a curved arrow to convert this intermediate into the more stable intermediate shown by resonance. / III C H Br Select Draw Rings More Erase C H Br Fulg SIW m by resonance. Feedback The step 2 intermediates are correct, but the curved arrows are incorrect. A carbocation delocalization will occur before \\( \ce (Br^{-}} \) adds to the \\(\ce{Varge ->} W) resonance structure. W Br
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


