Question: I need help with part C please explain. Below are the answers that I have gotten for Part A and Part B Which reagent is
I need help with part C please explain. Below are the answers that I have gotten for Part A and Part B
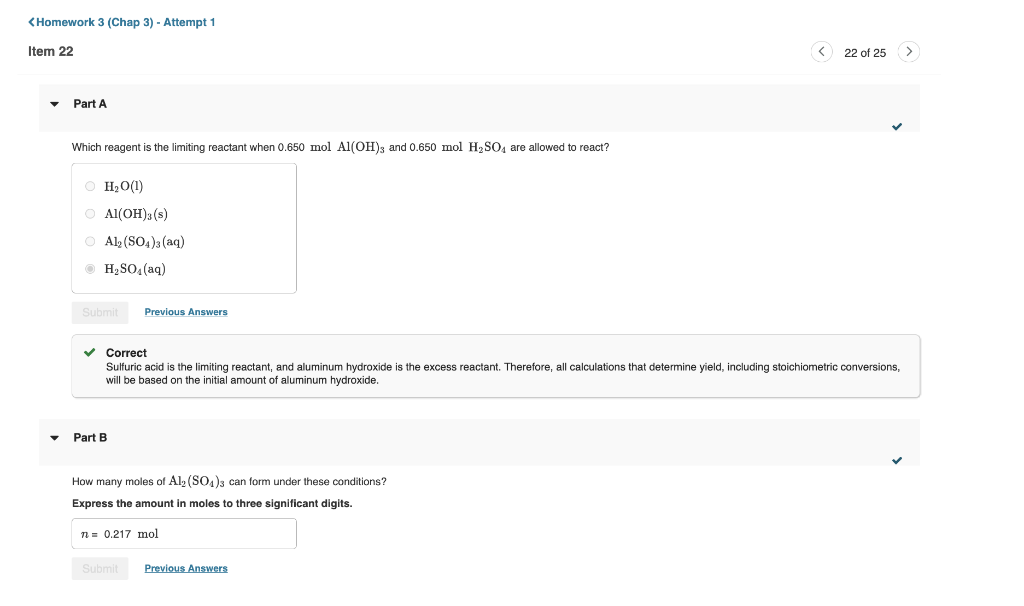
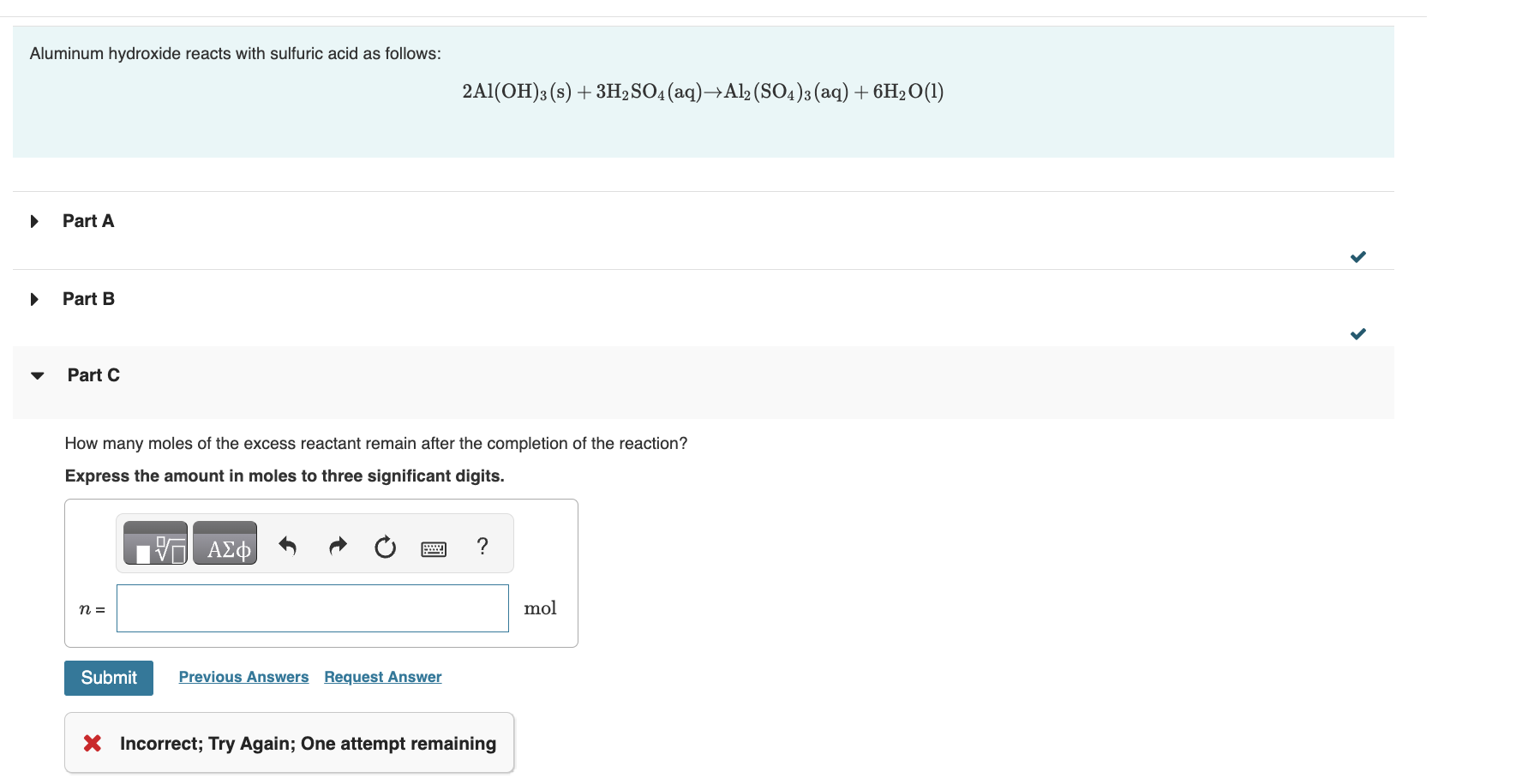
Which reagent is the limiting reactant when 0.650molAl(OH)3 and 0.650molH2SO4 are allowed to react? H2O(l)Al(OH)3(s)Al2(SO4)3(aq)H2SO4(aq) Correct Sulfuric acid is the limiting reactant, and aluminum hydroxide is the excess reactant. Therefore, all calculations that determine yield, including stoichiometric conversions, will be based on the initial amount of aluminum hydroxide. Part B How many moles of Al2(SO4)3 can form under these conditions? Express the amount in moles to three significant digits. Aluminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3(s)+3H2SO4(aq)Al2(SO4)3(aq)+6H2O(l) Part A Part B Part C How many moles of the excess reactant remain after the completion of the reaction? Express the amount in moles to three significant digits. * Incorrect; Try Again; One attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


