Question: I need help with question 1. i have attached everything done and instructions Data Treatment (45 pts.) 1. (6 pts.) Calculate the concentration of Cu2+

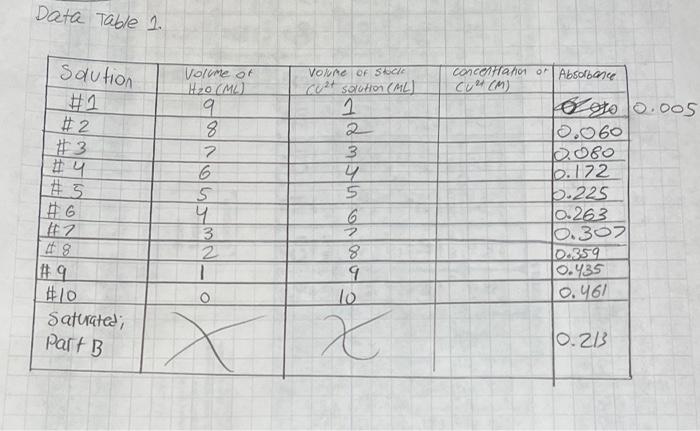


Data Treatment (45 pts.) 1. (6 pts.) Calculate the concentration of Cu2+ in the stock solution made from the 0.1M Cu2+ solution Data Table 1 saution concertant of Absorbance #2 Volume of H2O (MO) 9 8. 2 6 5 4 3 Vece f Sec cert solution (ML) 1 2 3 y 5 #3 25 oto 0.005 2.060 6.080 b. 172 5.225 10.263 10.307 10.359 0.435 0.461 #7 2 1 8 9 lo O #10 Saturated; Part B X 10.213 PROCEDURE CAUTION: WEAR SAFETY GOGGLES AND FOLLOW ALL STANDARD LABORATORY SAFETY PROCEDURES. PART A. BEER'S LAW 1. Refer to pages 41 - 43 and steps 1 - 14 for instructions on how to operate the PASCO spectrometer. Stop Solutions Obtain about 10 mL of 0.1 M copper(II)ion solution (i.e., the CuSO4). Pipet 10.0 mL into a 100.00-ml volumetric flask. Dilute to the mark with Dl water. Cover the opening with parafilm and carefully mix. 3. In a small beaker, prepare the first solution in Data Table 1. Use a Mohr pipet or a graduated cylinder to measure out the necessary volumes of the stock solution and deionized water. Cover the opening with parafilm and carefully mix. 4. Fill a cuvette two-thirds full with the solution prepared in step #3. In the hood, add 5 drops of concentrated ammonium hydroxide (NH4OH) to the solution in the cuvette. Mix thoroughly with a stirring rod. PART B. PREPARATION OF COPPER 1ODATE SOLUTION STEPS 6 AND 7 SHOULD BE DONE ONE WEEK IN ADVANCE. ALTERNATELY, YOUR INSTRUCTOR MAY CHOOSE TO ELIMINATE STEPS 6 AND 7 BY PROVIDING A SATURATED SOLUTION PREPARED FOR YOU. 7. Prepare the following solution in a beaker using separate pipets for each reagent. Pipet 5 mL of the 0.01 M Cu2 solution and 5 mL of 0.01 M iodate ion solution into the beaker. Let stand for 10 minutes. a 8. Line a funnel with filter paper as directed by your instructor. Filter the solution prepared in step #6. (Alternatively, centrifuge the sample to remove the precipitate.) 9. Fill a cuvette two-thirds full with the filtrate from step #7. Add 5 drops of ammonium hydroxide to the cuvette. Cover the opening with parafilm and carefully mix. Measure the absorbance at 610 nm of this saturated solution according to step 5 and record your measurement in Data Table 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


