Question: I need help with these questions What is the difference between (sigma) and (pi) bonds (select all that apply)? sigma bond can be made by
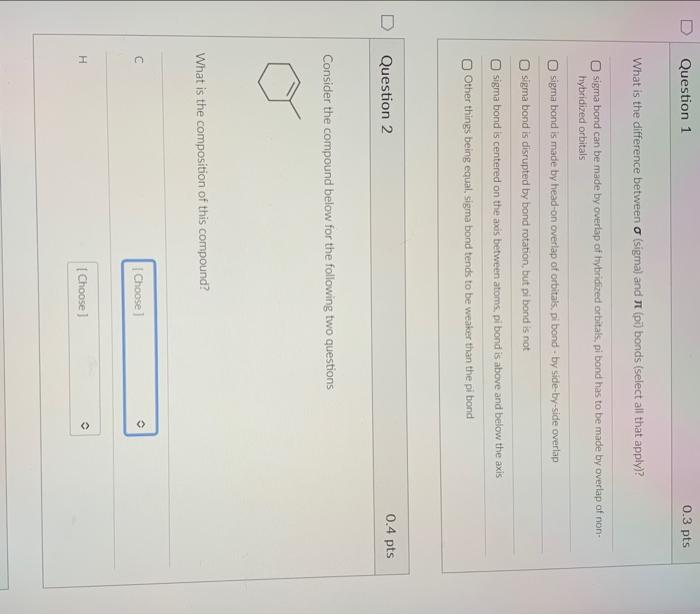
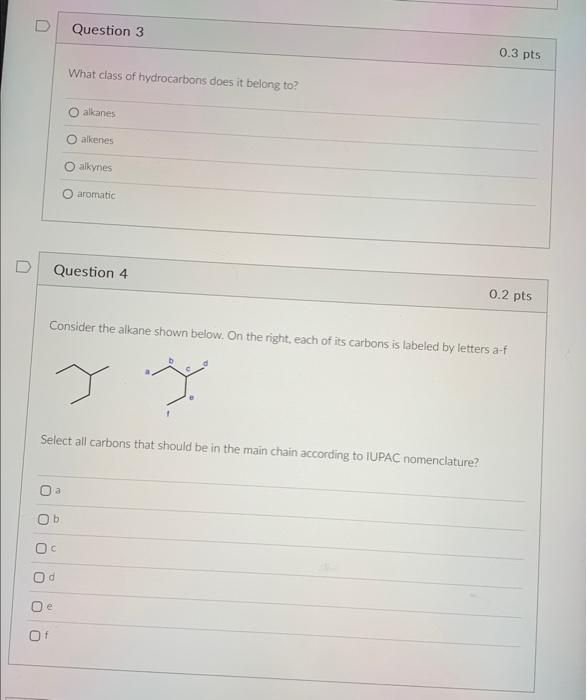
What is the difference between (sigma) and (pi) bonds (select all that apply)? sigma bond can be made by overlap of hybridized orbitats, pi bond has to be made by overlap of nonhybridized orbitals sigma bond is made by head-on overlap of orbitaks, pi bond - by side-by-side overlap. sigma bond is disrupted by bond rotation, but pi bond is not sigma bond is centered on the axos between atoms, pi bond is above and beiow the axis Other things being equal, sigma bond tends to be weaker than the pi bond Question 2 0.4pts Consider the compound below for the following two questions What is the composition of this compound? ference between (sigma) and (pi) bonds (select all that apply)? What class of hydrocarbons does it belong to? alkanes alkenes aikynes aromatic Question 4 Consider the alkane shown below. On the right, each of its carbons is labeled by letters a-f. Select all carbons that should be in the main chain according to IUPAC nomenclature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


