Question: i need help with these The data in the table below were obtained for the reaction: A+BP The order of the reaction in B is
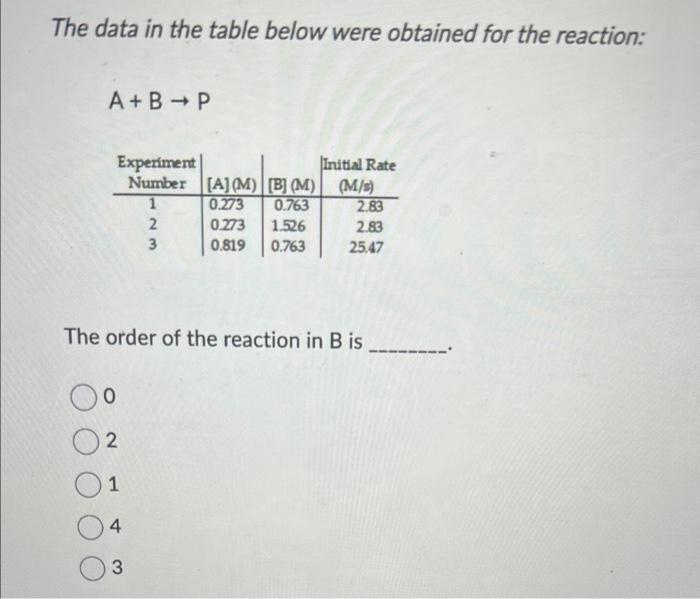

The data in the table below were obtained for the reaction: A+BP The order of the reaction in B is 0 2 1 4 Given the following rate law, how does the rate of reaction change if the concentration of X is doubled? Rate=k[X][Y]4 The rate of reaction will increase by a factor of 4 . The rate of reaction will increase by a factor of 5 . The rate of reaction will increase by a factor of 2 . The rate of reaction will remain unchanged. The rate of reaction will decrease by a factor of 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


