Question: i need help with this problem involving hybridization Pre-class Exercise - for each structure, identify Pre-class Exercise - determine which a) the hybridization of each
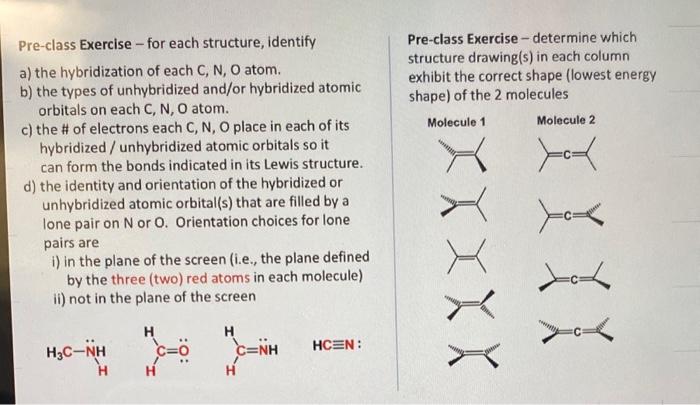
Pre-class Exercise - for each structure, identify Pre-class Exercise - determine which a) the hybridization of each C,N,O atom. structure drawing(s) in each column b) the types of unhybridized and/or hybridized atomic exhibit the correct shape (lowest energy orbitals on each C, N, O atom. c) the \# of electrons each C, N, O place in each of its hybridized/ unhybridized atomic orbitals so it can form the bonds indicated in its Lewis structure. d) the identity and orientation of the hybridized or unhybridized atomic orbital(s) that are filled by a lone pair on N or O. Orientation choices for lone pairs are i) in the plane of the screen (i.e., the plane defined by the three (two) red atoms in each molecule) ii) not in the plane of the screen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


