Question: I need help with this question. Constants for freezing-point depression and boiling-point elevation calculations at 1 atm: C G H 6 6 Ky value* Normal
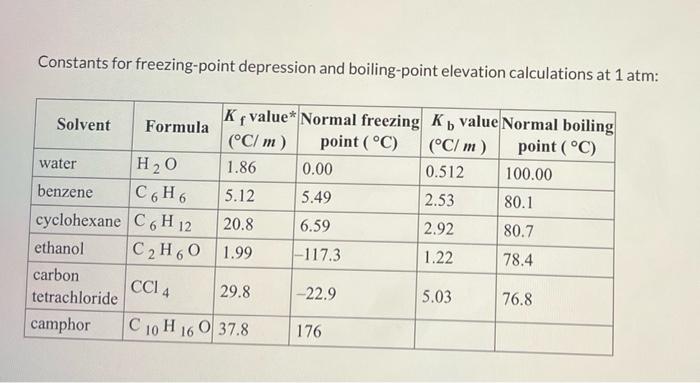

Constants for freezing-point depression and boiling-point elevation calculations at 1 atm: C G H 6 6 Ky value* Normal freezing K value Normal boiling Solvent Formula (C/m) point (C) (C/ m) point (C) water H20 1.86 0.00 0.512 100.00 benzene 5.12 5.49 2.53 80.1 cyclohexane C6H 12 20.8 6.59 2.92 80.7 ethanol C2H60 1.99 -117.3 1.22 78.4 carbon tetrachloride 29.8 -22.9 5.03 76.8 camphor C 10 H 16 0 37.8 176 CCI 4 A solution is made by dissolving 0.602 mol of nonelectrolyte solute in 887 g of benzene. Calculate the freezing point, Tr, and boiling point, 7. of the solution Constants can be found in the table of colligative constants. 1.80 Ti " incorrect 7. = 81.82 'C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


