Question: I need help with this question The below diene starting material is not symmetrical. Give a brief explanation for why we see this addition reaction
I need help with this question
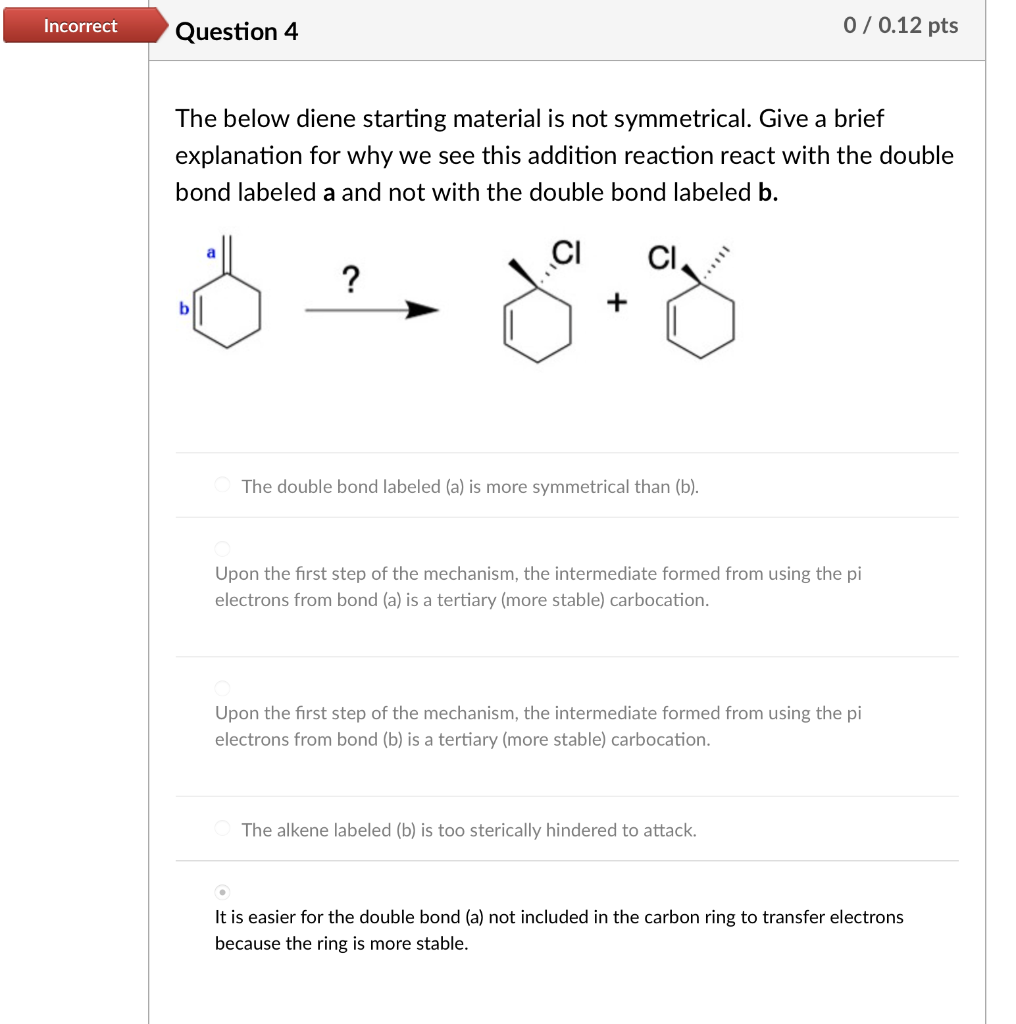
The below diene starting material is not symmetrical. Give a brief explanation for why we see this addition reaction react with the double bond labeled a and not with the double bond labeled b. The double bond labeled (a) is more symmetrical than (b). Upon the first step of the mechanism, the intermediate formed from using the pi electrons from bond (a) is a tertiary (more stable) carbocation. Upon the first step of the mechanism, the intermediate formed from using the pi electrons from bond (b) is a tertiary (more stable) carbocation. The alkene labeled (b) is too sterically hindered to attack. It is easier for the double bond (a) not included in the carbon ring to transfer electrons because the ring is more stable
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


