Question: I need helping explaining ny observations based on Le Chartlier's principle. What effect does temperature appear to have on the equilibrium of this system? How
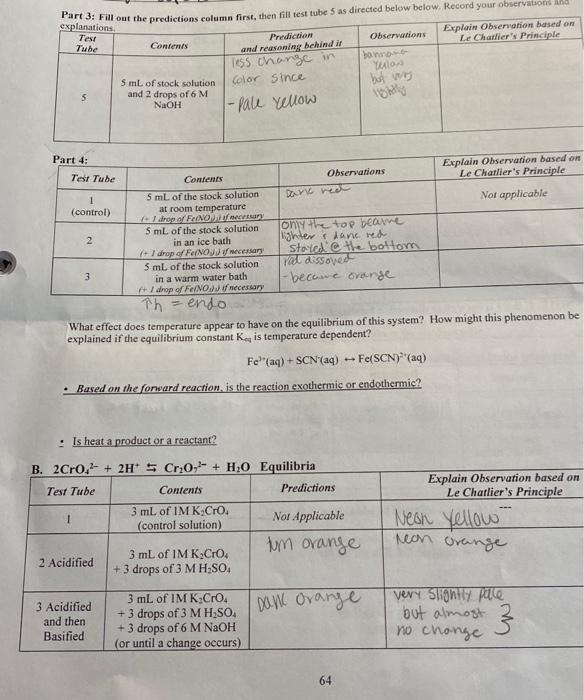
What effect does temperature appear to have on the equilibrium of this system? How might this phenomenon be explained if the equilibrium constant Ked is temperature dependent? Fe3(aq)+SCN(aq)Fe(SCN2(aq) - Based on the fonward reaction, is the reaction exothermic or endothermic? Is heat a product or a reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


