Question: I need it badly. Please answer asap Example 3.2 An ironmaking blast furnace produces hot metal of the following composition: 94% Fe, 2.2% Si, 3.8%
I need it badly. Please answer asap 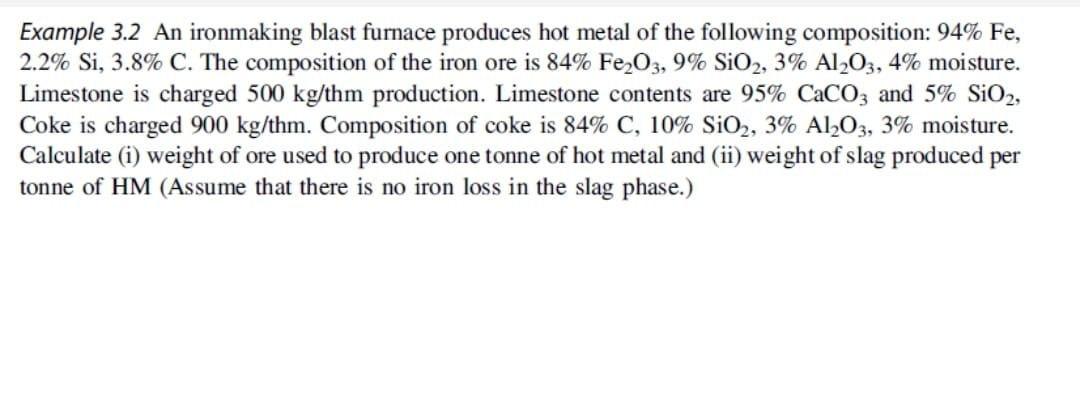
Example 3.2 An ironmaking blast furnace produces hot metal of the following composition: 94% Fe, 2.2% Si, 3.8% C. The composition of the iron ore is 84% Fe,O3, 9% SiO2, 3% Al2O3, 4% moisture. Limestone is charged 500 kg/thm production. Limestone contents are 95% CaCO3 and 5% S102, Coke is charged 900 kg/thm. Composition of coke is 84% C, 10% SiO2, 3% A1,03, 3% moisture. Calculate (i) weight of ore used to produce one tonne of hot metal and (ii) weight of slag produced per tonne of HM (Assume that there is no iron loss in the slag phase.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


