Question: I need it done in excel, please. 1. A series of experiments is prepared according to the following conditions: element Mass, 8 benzene 0 20
I need it done in excel, please.
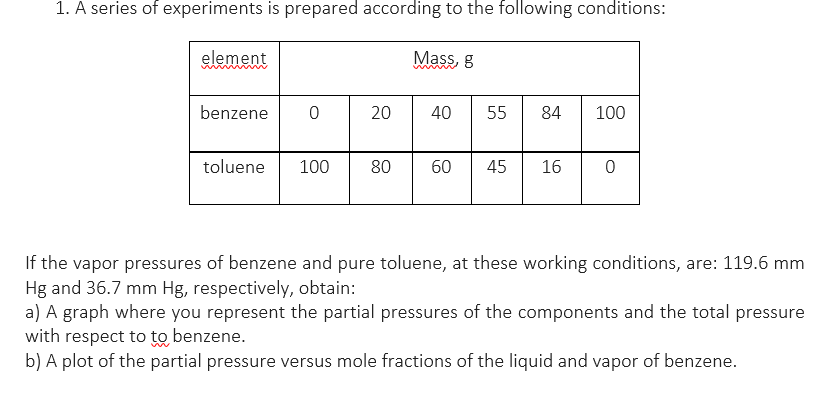
1. A series of experiments is prepared according to the following conditions: element Mass, 8 benzene 0 20 40 55 84 100 toluene 100 80 60 45 16 0 If the vapor pressures of benzene and pure toluene, at these working conditions, are: 119.6 mm Hg and 36.7 mm Hg, respectively, obtain: a) A graph where you represent the partial pressures of the components and the total pressure with respect to to benzene. b) A plot of the partial pressure versus mole fractions of the liquid and vapor of benzene
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


