Question: I need numer 2, 3 , 4 and 5 . 3. 1,10 - Phenanthroline forms a bright red complex with iron (II). The complex has

I need numer 2, 3 , 4 and 5
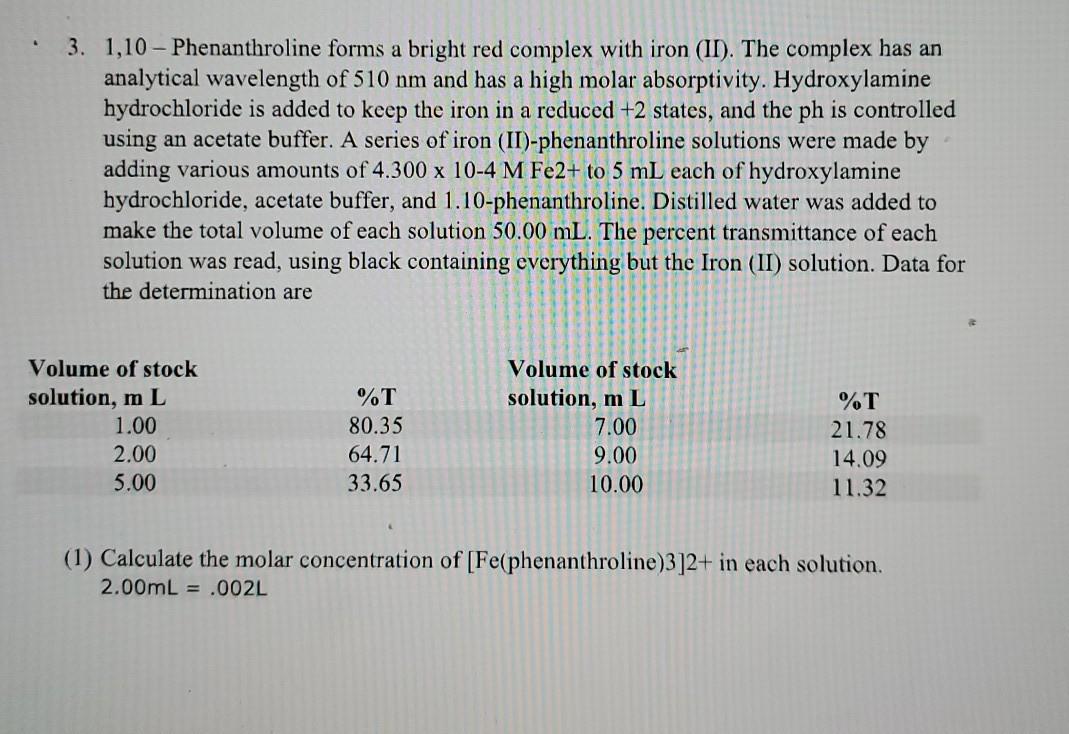
. 3. 1,10 - Phenanthroline forms a bright red complex with iron (II). The complex has an analytical wavelength of 510 nm and has a high molar absorptivity. Hydroxylamine hydrochloride is added to keep the iron in a reduced +2 states, and the ph is controlled using an acetate buffer. A series of iron (II)-phenanthroline solutions were made by adding various amounts of 4.300 x 10-4 M Fe2+ to 5 mL each of hydroxylamine hydrochloride, acetate buffer, and 1.10-phenanthroline. Distilled water was added to make the total volume of each solution 50.00 mL. The percent transmittance of each solution was read, using black containing everything but the Iron (II) solution. Data for the determination are Volume of stock solution, mL 1.00 2.00 5.00 %T 80.35 64.71 33.65 Volume of stock solution, m L 7.00 9.00 10.00 %T 21.78 14.09 11.32 (1) Calculate the molar concentration of [Fe(phenanthroline)3]2+ in each solution. 2.00mL = .002L (4.300*10^-4M)(.002L)=8.6*10^-7 mol 50.00mL = .05L, then use this, because this contains everything that has been added together/mixed, which would be the final volume. 8.6*10^-7 mol = 1.72*10^5M (2) Convert percent transmittance to equivalence absorbances, using Equation 4. (3) Prepare a Beer's law plot for [Fe(phenanthroline)3]2+, using the data obtained in (1) and (2). Draw a best straight line through the data points. (4) A solution containing an unknown amount of iron(III) was treated with hydroxylamine hydrocholoride, acetate buffer, and 1,10 - phenanthroline as described above. The percent transmittance of the sample at a= 510 nm was 52.4 when read against a blank containing all but the iron(II) solution. Determine the molar concentration of iron(II) in the unknown. (5) Calculate the molar absorptivity for [Fe(phenanthroline)3]2+. Assume a 1.00-cm path length
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


