Question: I need only #2 please, I believe it can be analyzed logically! Problem 4-5 (Level 2) A reactor is to be sized to carry out
I need only #2 please, I believe it can be analyzed logically!
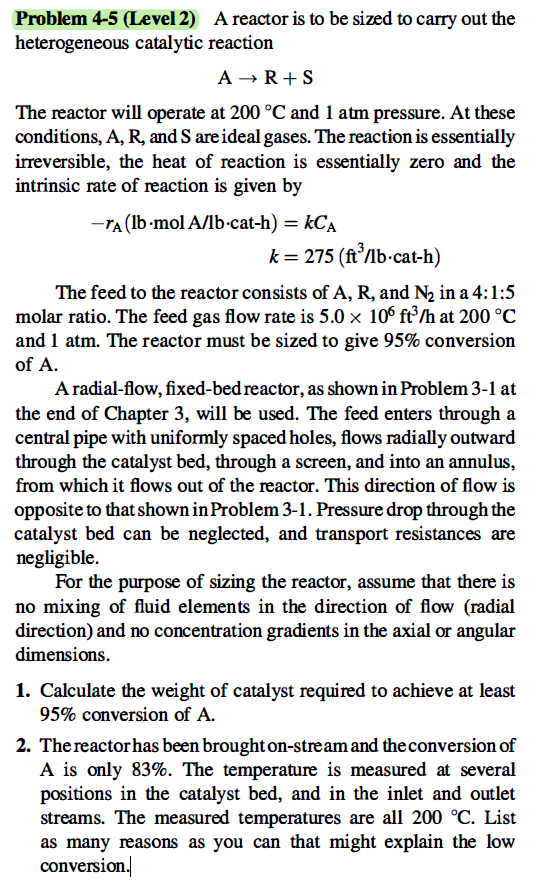
Problem 4-5 (Level 2) A reactor is to be sized to carry out the heterogeneous catalytic reaction A R+S The reactor will operate at 200 C and 1 atm pressure. At these conditions, A, R, and S are ideal gases. The reaction is essentially irreversible, the heat of reaction is essentially zero and the intrinsic rate of reaction is given by -ra (lb.mol A/lb-cat-h) = kCA k= 275 (ft/b-cat-h) The feed to the reactor consists of A, R, and N2 in a 4:1:5 molar ratio. The feed gas flow rate is 5.0 x 106 ft3/h at 200 C and 1 atm. The reactor must be sized to give 95% conversion of A. A radial-flow, fixed-bed reactor, as shown in Problem 3-1 at the end of Chapter 3, will be used. The feed enters through a central pipe with uniformly spaced holes, flows radially outward through the catalyst bed, through a screen, and into an annulus, from which it flows out of the reactor. This direction of flow is opposite to that shown in Problem 3-1. Pressure drop through the catalyst bed can be neglected, and transport resistances are negligible. For the purpose of sizing the reactor, assume that there is no mixing of fluid elements in the direction of flow (radial direction) and no concentration gradients in the axial or angular dimensions. 1. Calculate the weight of catalyst required to achieve at least 95% conversion of A. 2. The reactor has been broughton-stream and the conversion of A is only 83%. The temperature is measured at several positions in the catalyst bed, and in the inlet and outlet streams. The measured temperatures are all 200 C. List as many reasons as you can that might explain the low conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


