Question: I need some help with how to do this in excel. I have gone through every question on my own but each of my estimation
I need some help with how to do this in excel. I have gone through every question on my own but each of my estimation types is incredibly far off. The rectangle and trapezoid estimations are close, but the other two are magnitudes smaller. I'm very confused as I am certain I did everything right!
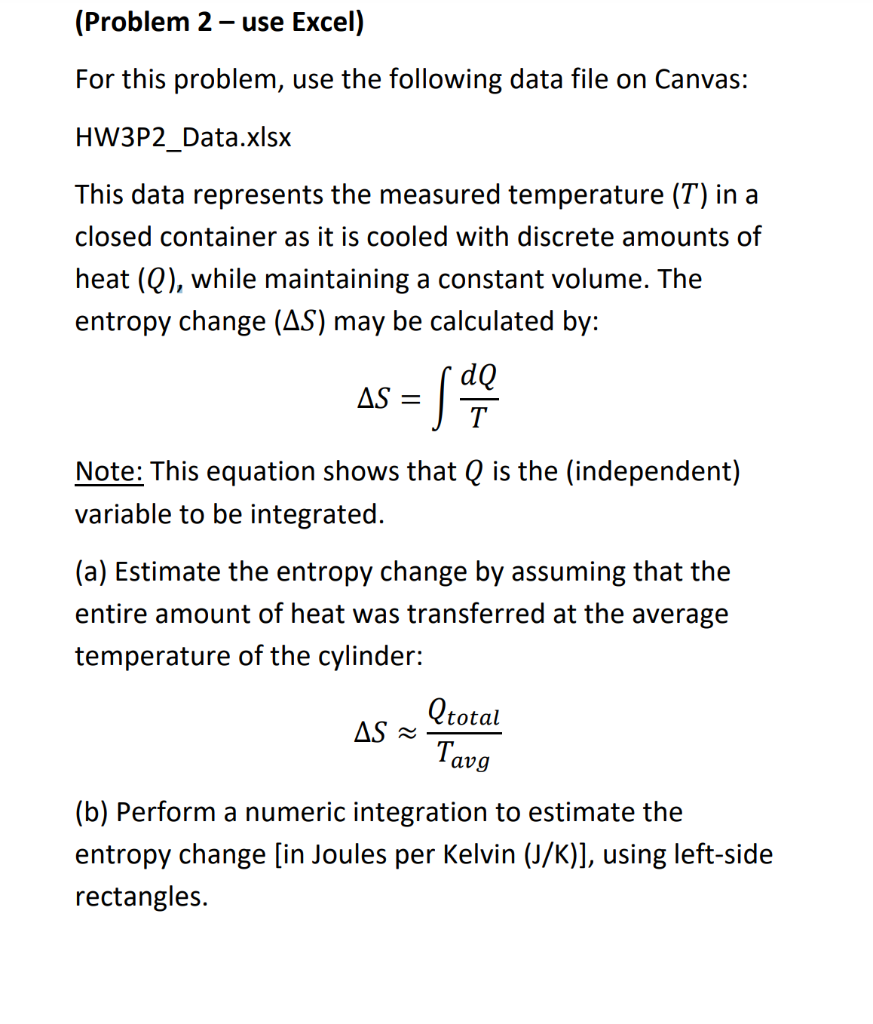
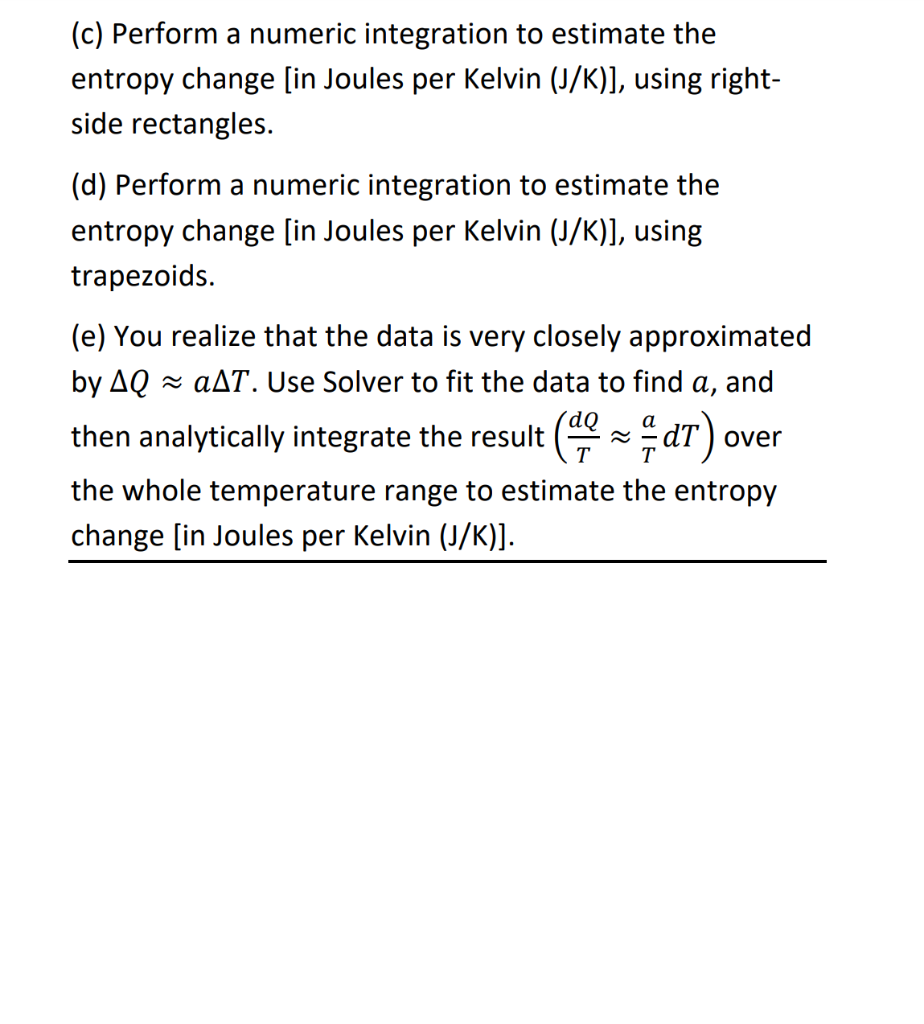
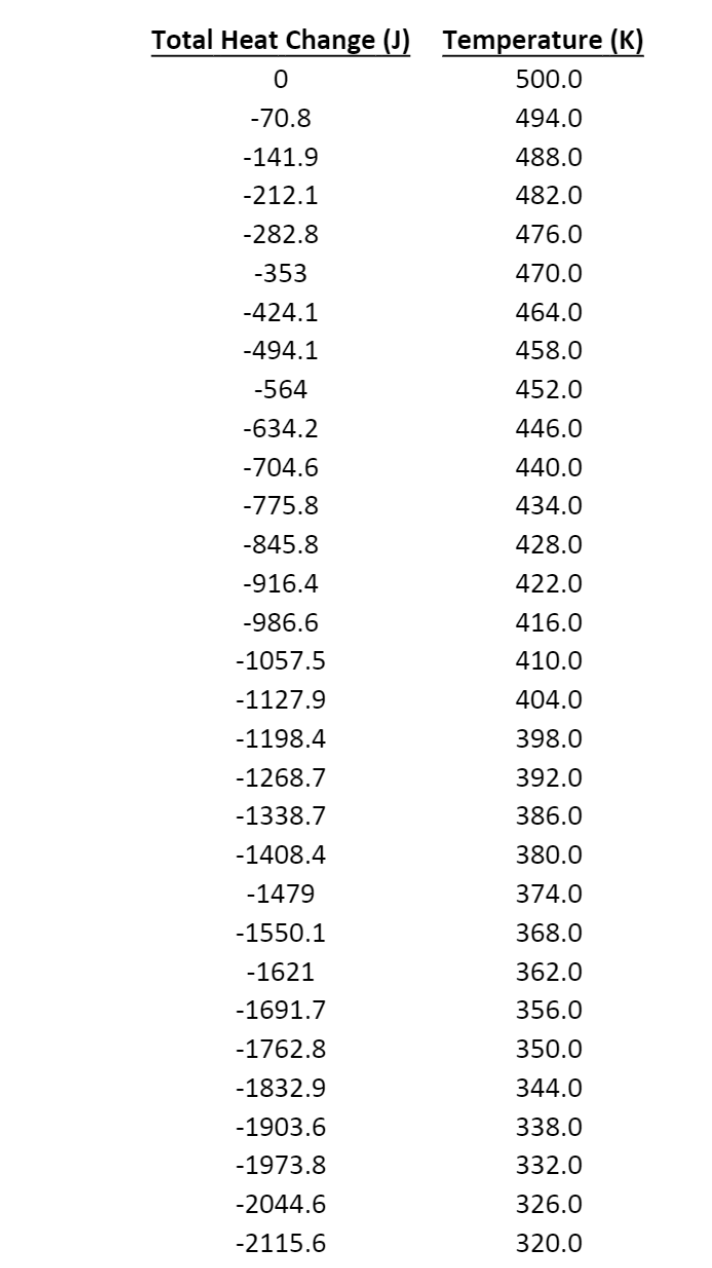
(Problem 2 - use Excel) For this problem, use the following data file on Canvas: HW3P2_Data.xlsx This data represents the measured temperature (T) in a closed container as it is cooled with discrete amounts of heat (Q), while maintaining a constant volume. The entropy change (AS) may be calculated by: dQ AS = Sao Note: This equation shows that Q is the (independent) variable to be integrated. (a) Estimate the entropy change by assuming that the entire amount of heat was transferred at the average temperature of the cylinder: (total AS Tavg (b) Perform a numeric integration to estimate the entropy change [in Joules per Kelvin (J/K)], using left-side rectangles. (c) Perform a numeric integration to estimate the entropy change [in Joules per Kelvin (J/K)], using right- side rectangles. (d) Perform a numeric integration to estimate the entropy change [in Joules per Kelvin (J/K)], using trapezoids. (e) You realize that the data is very closely approximated by AQ aAT. Use Solver to fit the data to find a, and over the whole temperature range to estimate the entropy change [in Joules per Kelvin (J/K)]. then analytically integrate the result (9* dT). Total Heat Change (1) 0 Temperature (K) 500.0 494.0 -70.8 -141.9 488.0 482.0 476.0 -212.1 -282.8 -353 -424.1 -494.1 470.0 464.0 458.0 -564 452.0 -634.2 -704.6 446.0 440.0 434.0 -775.8 -845.8 428.0 -916.4 422.0 416.0 410.0 -986.6 -1057.5 -1127.9 -1198.4 -1268.7 -1338.7 404.0 398.0 392.0 386.0 -1408.4 380.0 374.0 -1479 -1550.1 368.0 -1621 362.0 -1691.7 -1762.8 -1832.9 356.0 350.0 344.0 338.0 332.0 -1903.6 -1973.8 -2044.6 -2115.6 326.0 320.0 (Problem 2 - use Excel) For this problem, use the following data file on Canvas: HW3P2_Data.xlsx This data represents the measured temperature (T) in a closed container as it is cooled with discrete amounts of heat (Q), while maintaining a constant volume. The entropy change (AS) may be calculated by: dQ AS = Sao Note: This equation shows that Q is the (independent) variable to be integrated. (a) Estimate the entropy change by assuming that the entire amount of heat was transferred at the average temperature of the cylinder: (total AS Tavg (b) Perform a numeric integration to estimate the entropy change [in Joules per Kelvin (J/K)], using left-side rectangles. (c) Perform a numeric integration to estimate the entropy change [in Joules per Kelvin (J/K)], using right- side rectangles. (d) Perform a numeric integration to estimate the entropy change [in Joules per Kelvin (J/K)], using trapezoids. (e) You realize that the data is very closely approximated by AQ aAT. Use Solver to fit the data to find a, and over the whole temperature range to estimate the entropy change [in Joules per Kelvin (J/K)]. then analytically integrate the result (9* dT). Total Heat Change (1) 0 Temperature (K) 500.0 494.0 -70.8 -141.9 488.0 482.0 476.0 -212.1 -282.8 -353 -424.1 -494.1 470.0 464.0 458.0 -564 452.0 -634.2 -704.6 446.0 440.0 434.0 -775.8 -845.8 428.0 -916.4 422.0 416.0 410.0 -986.6 -1057.5 -1127.9 -1198.4 -1268.7 -1338.7 404.0 398.0 392.0 386.0 -1408.4 380.0 374.0 -1479 -1550.1 368.0 -1621 362.0 -1691.7 -1762.8 -1832.9 356.0 350.0 344.0 338.0 332.0 -1903.6 -1973.8 -2044.6 -2115.6 326.0 320.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


