Question: I need some help with my Chemical Equilibrium Pre-Lab assignment Pre-Lab Assignment NAME NOTE: The absorbance data used here are for illustrative purposes. The absorbance
I need some help with my Chemical Equilibrium Pre-Lab assignment
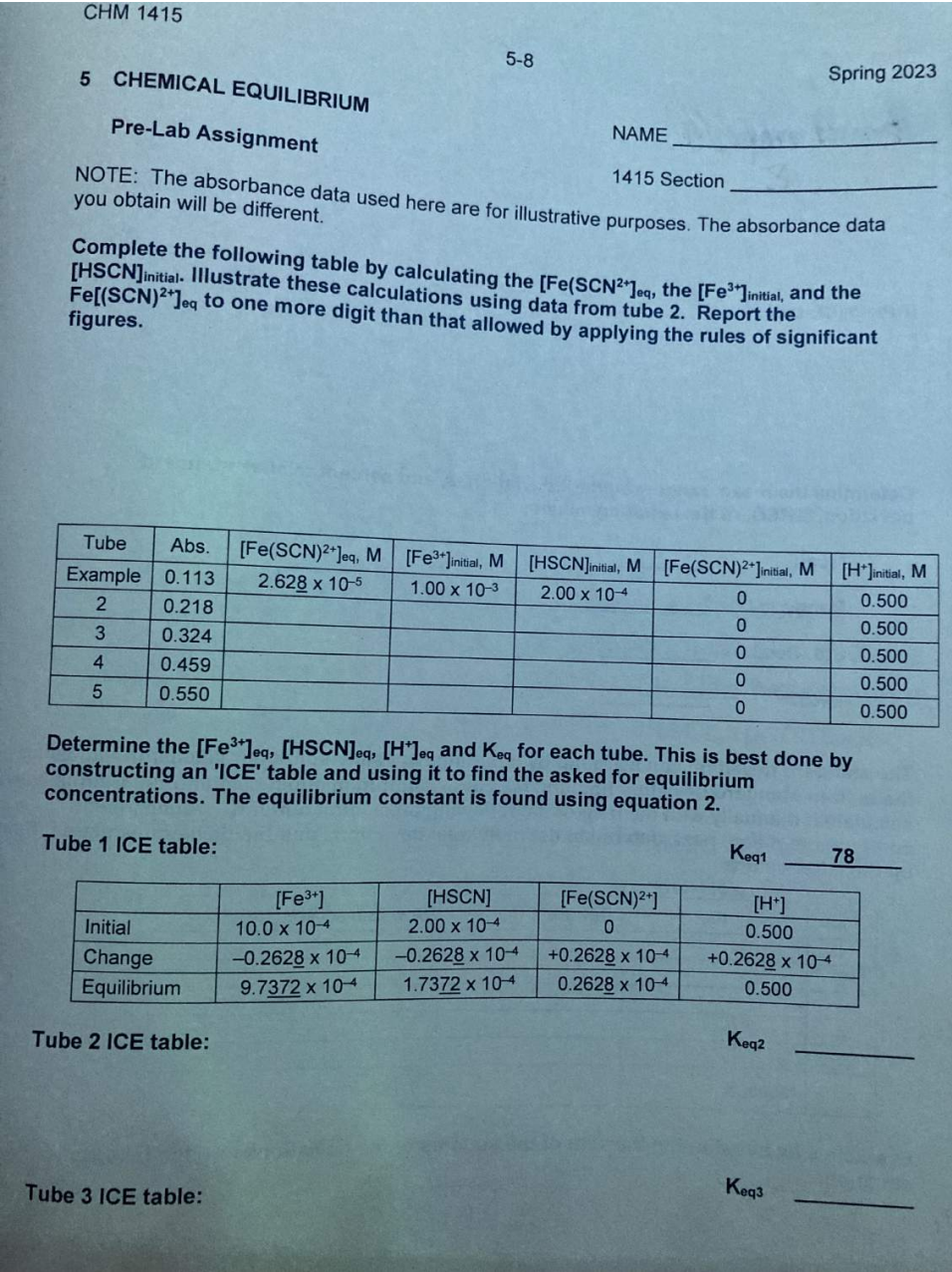
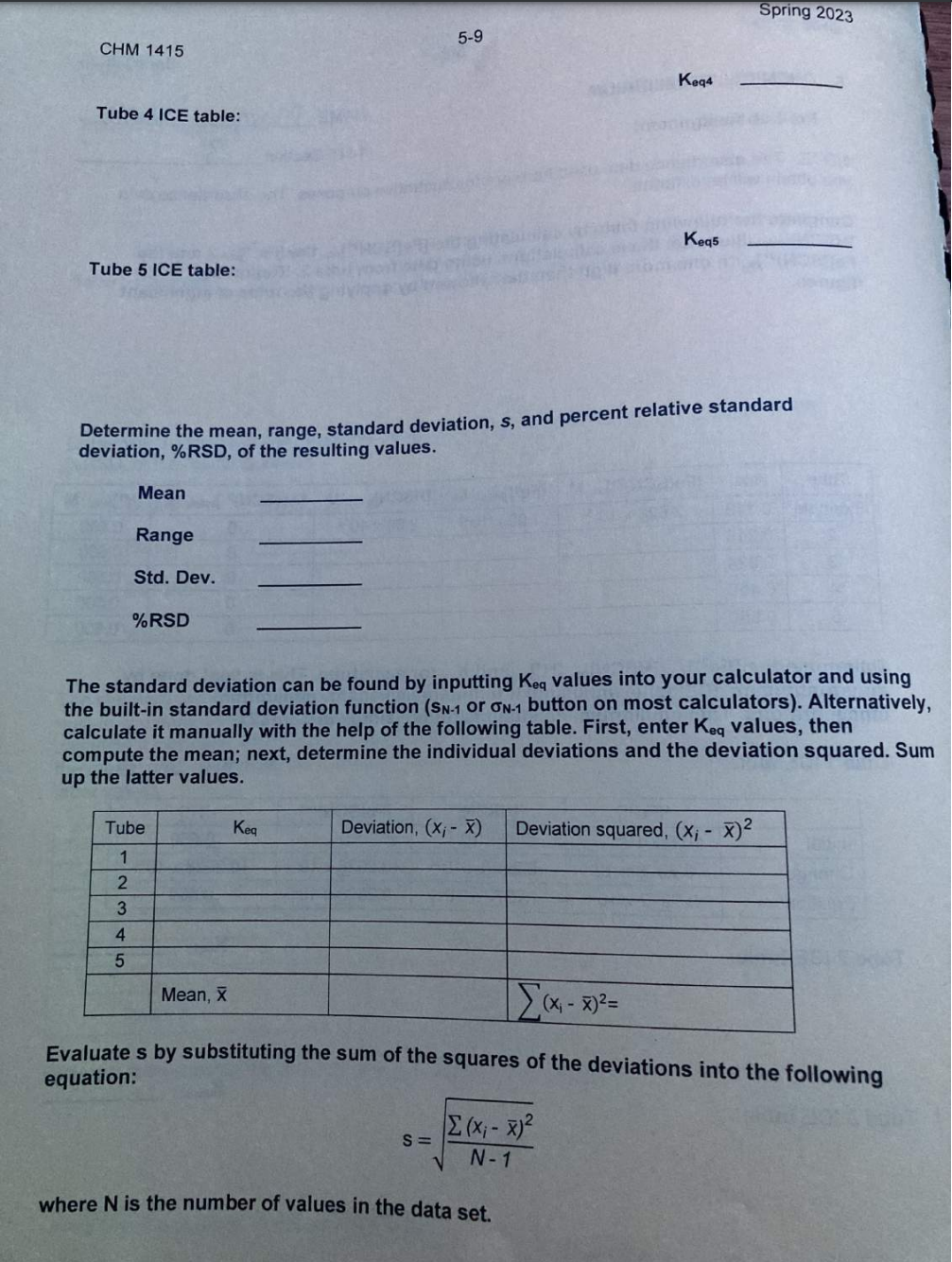
Pre-Lab Assignment NAME NOTE: The absorbance data used here are for illustrative purposes. The absorbance data you obtain will be different. Complete the following table by calculating the [FeSCN2+]eq, the [Fe3]initial, and the [HSCN] initial. Illustrate these calculations using data from tube 2. Report the Fe[(SCN Determine the [Fe3+]eq, [HSCN]eq[H+]eq and Keq for each tube. This is best done by constructing an 'ICE' table and using it to find the asked for equilibrium concentrations. The equilibrium constant is found using equation 2. Tube 1 ICE table: Keq1 Tube 2 ICE table: Tube 3 ICE table: Keq3 Tube 4 ICE table: Keq5 Tube 5 ICE table: Determine the mean, range, standard deviation, s, and percent relative standard deviation, \%RSD, of the resulting values. Mean Range Std. Dev. \%RSD The standard deviation can be found by inputting Keq values into your calculator and using the built-in standard deviation function (s sN1 or N1 button on most calculators). Alternatively, calculate it manually with the help of the following table. First, enter Keq values, then compute the mean; next, determine the individual deviations and the deviation squared. Sum up the latter values. Evaluate s by substituting the sum of the squares of the deviations into the following equation: s=N1(xix)2 where N is the number of values in the data set. Pre-Lab Assignment NAME NOTE: The absorbance data used here are for illustrative purposes. The absorbance data you obtain will be different. Complete the following table by calculating the [FeSCN2+]eq, the [Fe3]initial, and the [HSCN] initial. Illustrate these calculations using data from tube 2. Report the Fe[(SCN Determine the [Fe3+]eq, [HSCN]eq[H+]eq and Keq for each tube. This is best done by constructing an 'ICE' table and using it to find the asked for equilibrium concentrations. The equilibrium constant is found using equation 2. Tube 1 ICE table: Keq1 Tube 2 ICE table: Tube 3 ICE table: Keq3 Tube 4 ICE table: Keq5 Tube 5 ICE table: Determine the mean, range, standard deviation, s, and percent relative standard deviation, \%RSD, of the resulting values. Mean Range Std. Dev. \%RSD The standard deviation can be found by inputting Keq values into your calculator and using the built-in standard deviation function (s sN1 or N1 button on most calculators). Alternatively, calculate it manually with the help of the following table. First, enter Keq values, then compute the mean; next, determine the individual deviations and the deviation squared. Sum up the latter values. Evaluate s by substituting the sum of the squares of the deviations into the following equation: s=N1(xix)2 where N is the number of values in the data set
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


