Question: I need step by step solution with an explanation, please. I appreciate any help you can provide. Calculate the standard enthalpy of formation of acetylene
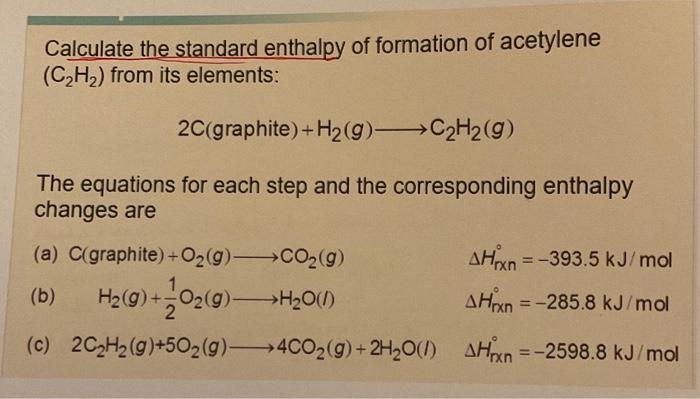
Calculate the standard enthalpy of formation of acetylene (C2H2) from its elements: 2C(graphite)+H2(g)C2H2(g) The equations for each step and the corresponding enthalpy changes are (a) C (graphite) +O2(g)CO2(g) Hrn=393.5kJ/mol (b) H2(g)+21O2(g)H2O(l)Hxn=285.8kJ/mol (c) 2C2H2(g)+5O2(g)4CO2(g)+2H2O(I)Hrn=2598.8kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


