Question: 9 mol P4010 reacts with 51 mol HO according to the equation below: P4010 + 6HO 4H3PO4 How many moles of H3PO4 form from
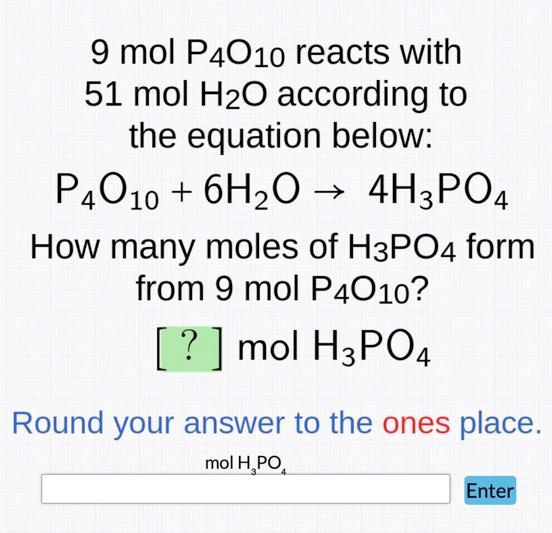
9 mol P4010 reacts with 51 mol HO according to the equation below: P4010 + 6HO 4H3PO4 How many moles of H3PO4 form from 9 mol P4010? [?] mol H3PO4 Round your answer to the ones place. mol HPO Enter
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
The balanced chemical equation shows that 1 mole ... View full answer

Get step-by-step solutions from verified subject matter experts


