Question: I need the answer as soon as possible (b) Use appropriate Walsh diagram(s) (from pages 54 - 56 of the Symmetry Guide) and molecular orbital
I need the answer as soon as possible 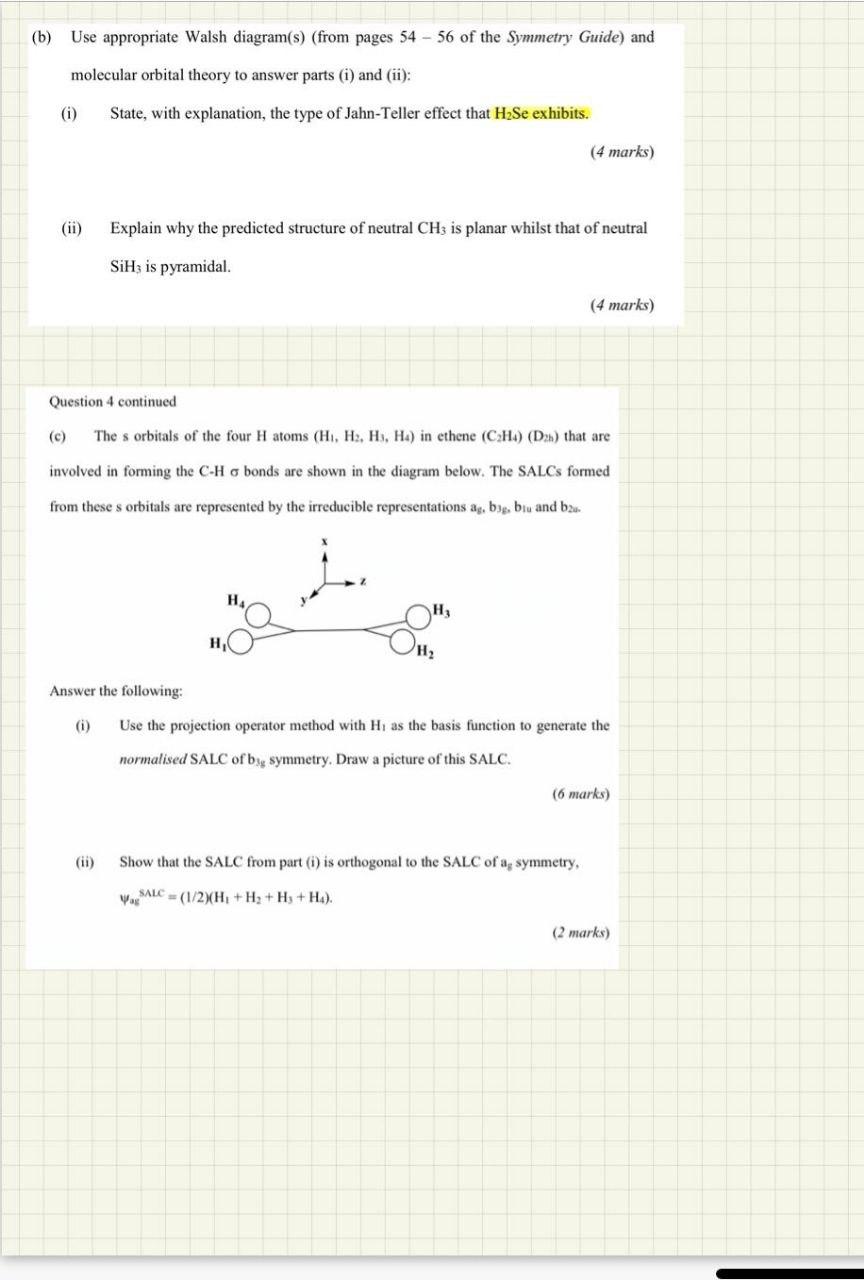
(b) Use appropriate Walsh diagram(s) (from pages 54 - 56 of the Symmetry Guide) and molecular orbital theory to answer parts (i) and (ii): (i) State, with explanation, the type of Jahn-Teller effect that H2Se exhibits. (4 marks) (ii) Explain why the predicted structure of neutral CH3 is planar whilst that of neutral SiHz is pyramidal. (4 marks) Question 4 continued (c) The s orbitals of the four H atoms (H1, H3, H3, Ha) in ethene (CHA) (D2) that are involved in forming the C-H o bonds are shown in the diagram below. The SALCs formed from these s orbitals are represented by the irreducible representations as, big, blu and be H H Answer the following: (i) Use the projection operator method with Hi as the basis function to generate the normalised SALC of big symmetry. Draw a picture of this SALC. (6 marks) (ii) Show that the SALC from part (1) is orthogonal to the SALC of asymmetry, VASSALC = (1/2)(H + H2 + H3 + Ha). (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


