Question: I need the answer as soon as possible Example-1.3) Measurment of Molecular Diffusivity The molecular diffusivity of carbon tetrachloride (A) in air (B) will be
I need the answer as soon as possible 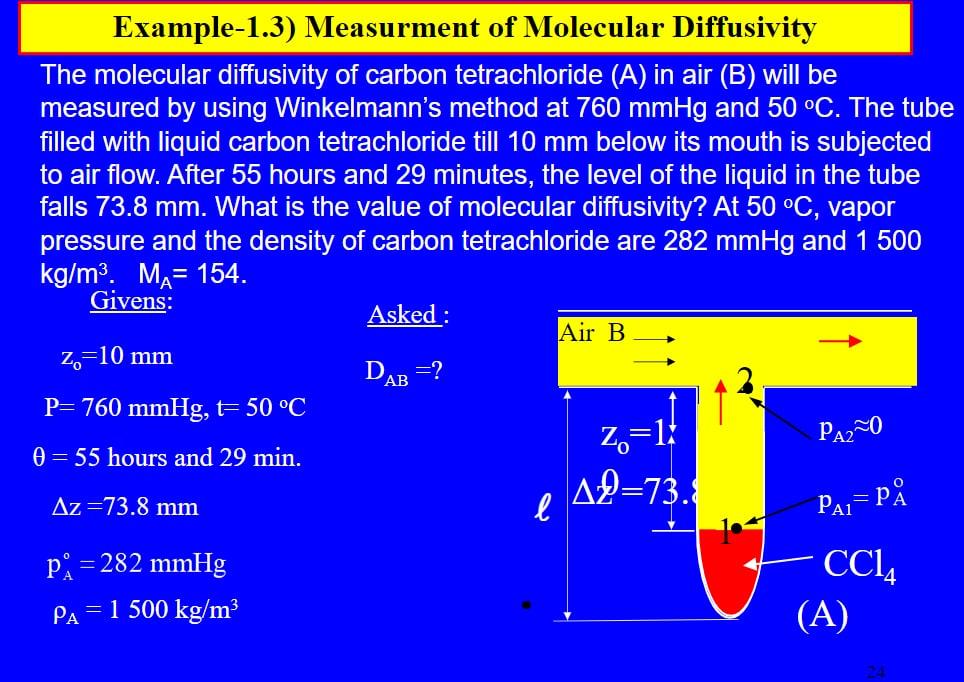
Example-1.3) Measurment of Molecular Diffusivity The molecular diffusivity of carbon tetrachloride (A) in air (B) will be measured by using Winkelmann's method at 760 mmHg and 50 C. The tube filled with liquid carbon tetrachloride till 10 mm below its mouth is subjected to air flow. After 55 hours and 29 minutes, the level of the liquid in the tube falls 73.8 mm. What is the value of molecular diffusivity? At 50 C, vapor pressure and the density of carbon tetrachloride are 282 mmHg and 1 500 kg/m. Ma= 154. Givens: Asked : Air B z=10 mm DAB =? P= 760 mmHg, t= 50 C Z=1 PA20 0 = 55 hours and 29 min. A2=73. Az=73.8 mm l PAL=P P8 = 282 mmHg PA= 1 500 kg/m3 CC, (A)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


