Question: I need the answer as soon as possible Q.3. Formaldehyde is produced by dehydrogenation of methanol. CH,OH HCHO + H2 The single pass conversion is
I need the answer as soon as possible 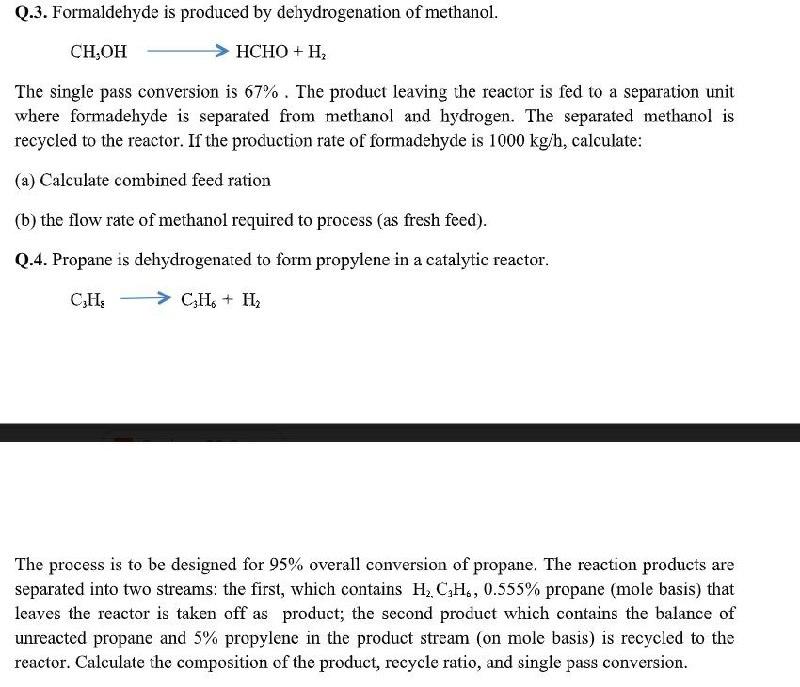
Q.3. Formaldehyde is produced by dehydrogenation of methanol. CH,OH HCHO + H2 The single pass conversion is 67%. The product leaving the reactor is fed to a separation unit where formadehyde is separated from methanol and hydrogen. The separated methanol is recycled to the reactor. If the production rate of formadehyde is 1000 kg/h, calculate: (a) Calculate combined feed ration (b) the flow rate of methanol required to process (as fresh feed). Q.4. Propane is dehydrogenated to form propylene in a catalytic reactor. CHE C3H + H2 The process is to be designed for 95% overall conversion of propane. The reaction products are separated into two streams: the first, which contains H, CH, 0.555% propane (mole basis) that leaves the reactor is taken off as product; the second product which contains the balance of unreacted propane and 5% propylene in the product stream (on mole basis) is recycled to the reactor. Calculate the composition of the product, recycle ratio, and single pass conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


