Question: The aims of this experiment are as follow: Calculate the volume % of each of the following component based on the information in the
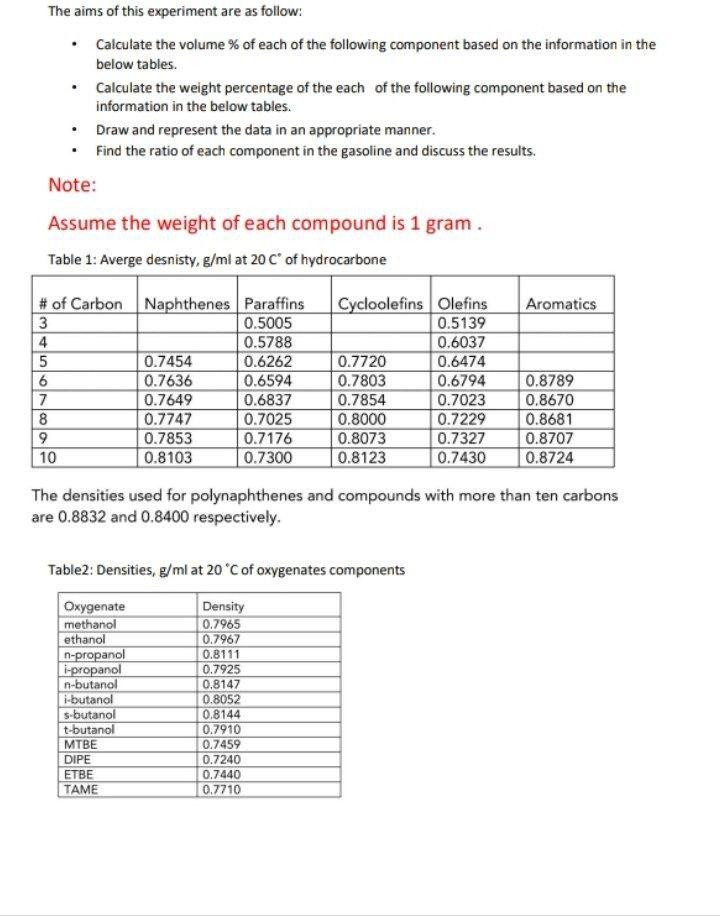
The aims of this experiment are as follow: Calculate the volume % of each of the following component based on the information in the below tables. Calculate the weight percentage of the each of the following component based on the information in the below tables. Draw and represent the data in an appropriate manner. Find the ratio of each component in the gasoline and discuss the results. Note: Assume the weight of each compound is 1 gram. Table 1: Averge desnisty, g/ml at 20 C of hydrocarbone 7 8 9 10 # of Carbon Naphthenes Paraffins 3 0.5005 4 0.5788 5 0.6262 6 0.6594 0.6837 0.7025 0.7176 0.7300 Oxygenate methanol ethanol 0.7454 0.7636 0.7649 0.7747 0.7853 0.8103 n-propanol i-propanol n-butanol i-butanol s-butanol t-butanol MTBE DIPE ETBE TAME Table2: Densities, g/ml at 20 C of oxygenates components Density 0.7965 0.7967 0.8111 0.7925 0.8147 0.8052 Cycloolefins Olefins 0.5139 0.8144 0.7910 0.7459 0.7720 0.7803 0.7854 0.8000 The densities used for polynaphthenes and compounds with more than ten carbons are 0.8832 and 0.8400 respectively. 0.7240 0.7440 0.7710 0.8073 0.8123 0.6037 0.6474 0.6794 0.7023 0.7229 0.7327 0.7430 Aromatics 0.8789 0.8670 0.8681 0.8707 0.8724
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
To solve this problem we need to follow the steps outlined in the aims 1 Calculate the volume percen... View full answer

Get step-by-step solutions from verified subject matter experts


