Question: I need the answer for this question pleas. A crystal of chalcanthite (CuSO45H2O) dissolves in a large tank of pure water according to the following
I need the answer for this question pleas.
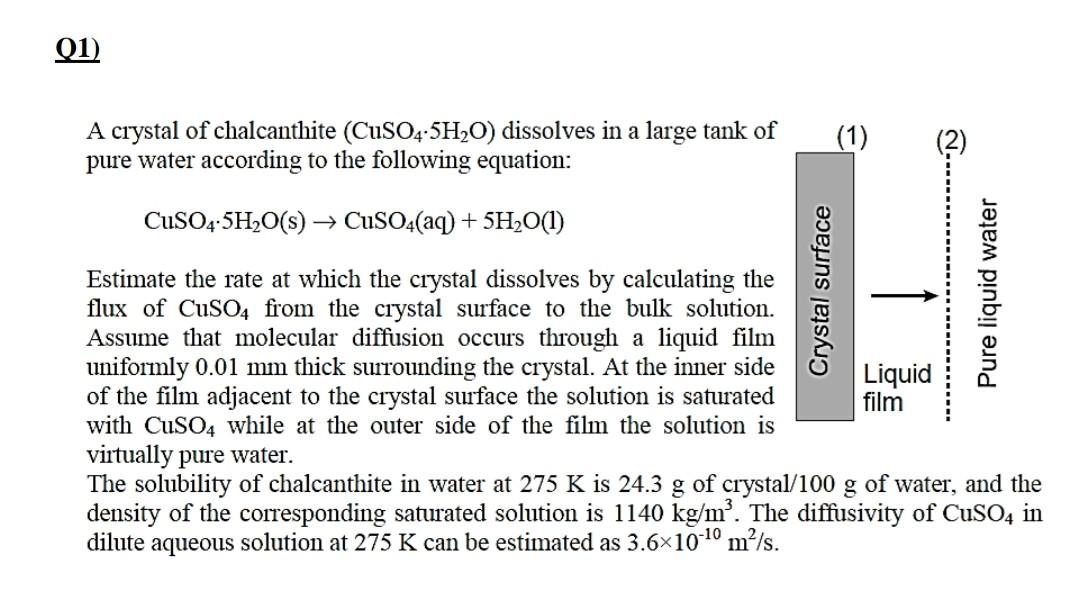
A crystal of chalcanthite (CuSO45H2O) dissolves in a large tank of pure water according to the following equation: CuSO45H2O(s)CuSO4(aq)+5H2O(l) Estimate the rate at which the crystal dissolves by calculating the flux of CuSO4 from the crystal surface to the bulk solution. Assume that molecular diffusion occurs through a liquid film uniformly 0.01mm thick surrounding the crystal. At the inner side of the film adjacent to the crystal surface the solution is saturated with CuSO4 while at the outer side of the film the solution is virtually pure water. The solubility of chalcanthite in water at 275K is 24.3g of crystal/100 g of water, and the density of the corresponding saturated solution is 1140kg/m3. The diffusivity of CuSO4 in dilute aqueous solution at 275K can be estimated as 3.61010m2/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


