Question: I need the answer for this question please. An absorption column, operated at 1.0atm and 300K, is used to remove species A from a gaseous
I need the answer for this question please.
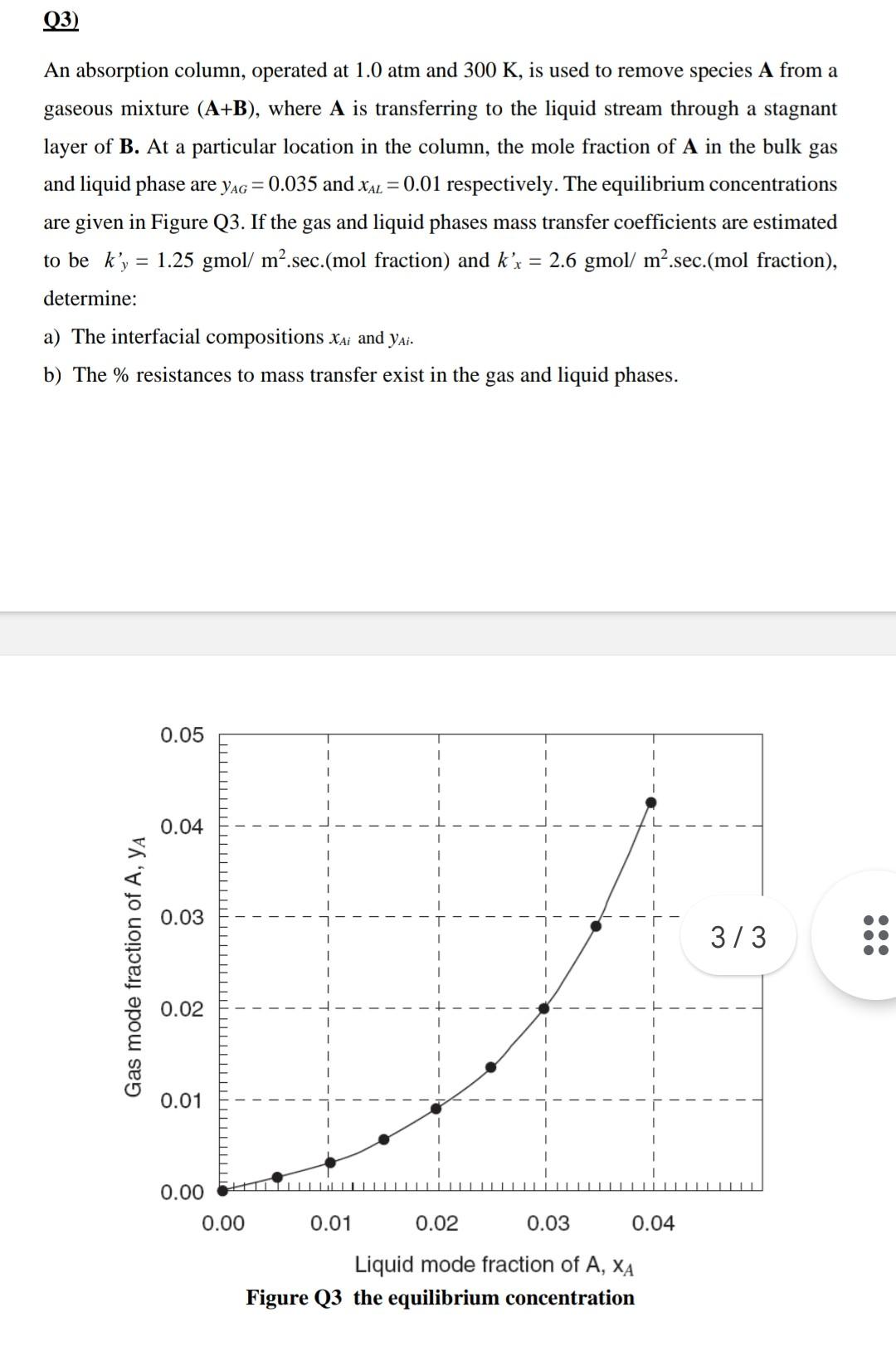
An absorption column, operated at 1.0atm and 300K, is used to remove species A from a gaseous mixture (A+B), where A is transferring to the liquid stream through a stagnant layer of B. At a particular location in the column, the mole fraction of A in the bulk gas and liquid phase are yAG=0.035 and xAL=0.01 respectively. The equilibrium concentrations are given in Figure Q3. If the gas and liquid phases mass transfer coefficients are estimated to be ky=1.25gmol/m2.sec.(mol fraction) and kx=2.6gmol/m2.sec.(mol fraction), determine: a) The interfacial compositions xAi and yAi. b) The % resistances to mass transfer exist in the gas and liquid phases
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


