Question: I need the correct solution Exercise 2. Consider the following liquid-phase elementary reversible chemical reaction: k1ABk2 where k1=0.751/s and k2=0.051/s. Calculate the time required to
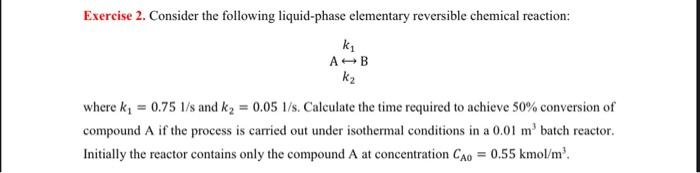
Exercise 2. Consider the following liquid-phase elementary reversible chemical reaction: k1ABk2 where k1=0.751/s and k2=0.051/s. Calculate the time required to achieve 50% conversion of compound A if the process is carried out under isothermal conditions in a 0.01m3 batch reactor. Initially the reactor contains only the compound A at concentration CA0=0.55kmol/m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


