Question: I need the delta H and double checking on chart The process path is broken into seven steps below; those steps facilitate the calculation of
I need the delta H and double checking on chart
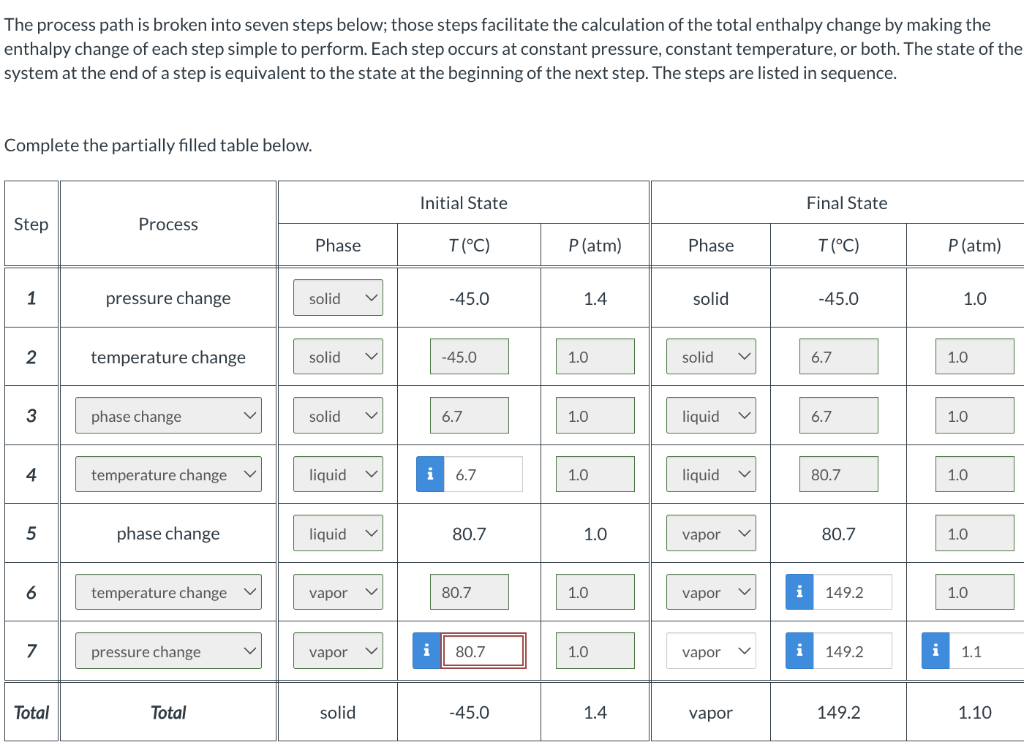
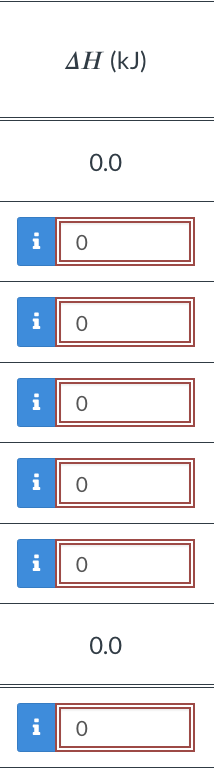
The process path is broken into seven steps below; those steps facilitate the calculation of the total enthalpy change by making the enthalpy change of each step simple to perform. Each step occurs at constant pressure, constant temperature, or both. The state of the system at the end of a step is equivalent to the state at the beginning of the next step. The steps are listed in sequence. Complete the partially filled table below. H(kJ) 0.0 i 0 i 0 i 0 i 0 i 0 0.0 i 0 The process path is broken into seven steps below; those steps facilitate the calculation of the total enthalpy change by making the enthalpy change of each step simple to perform. Each step occurs at constant pressure, constant temperature, or both. The state of the system at the end of a step is equivalent to the state at the beginning of the next step. The steps are listed in sequence. Complete the partially filled table below. H(kJ) 0.0 i 0 i 0 i 0 i 0 i 0 0.0 i 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


