Question: I need the solution to exercises 1, 2 and 3. thank you CSTR, cascade of CSTRs Exercise 1. Irreversible chemical reaction AB of the first
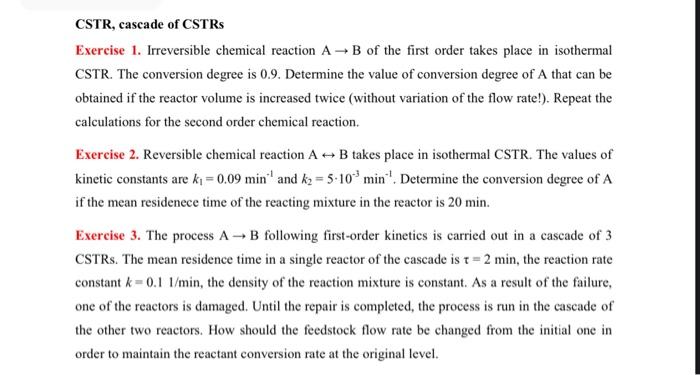
CSTR, cascade of CSTRs Exercise 1. Irreversible chemical reaction AB of the first order takes place in isothermal CSTR. The conversion degree is 0.9 . Determine the value of conversion degree of A that can be obtained if the reactor volume is increased twice (without variation of the flow rate!). Repeat the calculations for the second order chemical reaction. Exercise 2. Reversible chemical reaction AB takes place in isothermal CSTR. The values of kinetic constants are k1=0.09min1 and k2=5103min1. Determine the conversion degree of A if the mean residenece time of the reacting mixture in the reactor is 20min. Exercise 3. The process AB following first-order kinetics is carried out in a cascade of 3 CSTRs. The mean residence time in a single reactor of the cascade is =2min, the reaction rate constant k=0.11/min, the density of the reaction mixture is constant. As a result of the failure, one of the reactors is damaged. Until the repair is completed, the process is run in the cascade of the other two reactors. How should the feedstock flow rate be changed from the initial one in order to maintain the reactant conversion rate at the original level
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


