Question: I need to find the unkown compound in this lab can someone find it? what is the unknown compound of this lab? the last picture


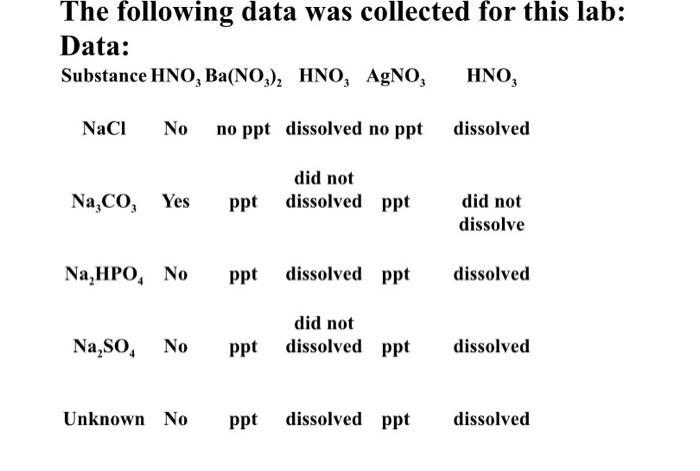
AP Chemistry Identification of an Unknown Lab Purpose: This experiment emphasizes the importance of making observations and inferences from those observations. Through the observations made in this lab, and comparing the reactions of known substances to those of an unknown substance, the identity of the unknown substance can be determined. Accurate analysis of data is necessary for success. Procedure: The results for three different tests will be given for the testing of the substances. The three tests are testing for gas evolution, testing with barium nitrate, and testing with silver nitrate. Testing for Gas Evolution 1. A pea-sized, solid sample of the unknown sample was placed into a test tube. Pea-sized, solid samples of each of the following samples: NaCl, Na,Co,, Na HPO, and Na So were placed into separate test tubes, 2.5 drops of HNO, solution were added to each of the five test tubes. 3. The test tubes were observed. If gas bubbles were produced this was recorded in the data table as yes and no if no bubbles were generated Dissolving the Unknown Compound 1. A 100ml graduated cylinder and a 400mL beaker were thoroughly washed with distilled water 2. A pea-sized, solid portion of the unknown was placed in the beaker then 200mL of distilled water were added from the graduated cylinder. The solution was stirred until the unknown completely dissolved. 3. This solution was set aside. Testing with Barium Nitrate 1. 20 drops of the known solutions (see above) were placed into 4 separate test tubes. 2. 20 drops of the unknown solution were placed into a 5 test tube. 3. 3 drops of NH, solution were added to each of the 5 test tubes. This step was done to make each of the solutions basic. 4. 5 drops of Ba(NO), solution were added to each of the 5 test tubes. The contents of each test tube was stirred with a stirring rod and observed for the formation of a precipitate. If a precipitate formed in a test tube, this was recorded in the data table as ppt. If no precipitate was formed in the test tube, this was recorded as no ppt 5. To only the test tubes in which a precipitate was formed, 10 drops of HNO, solution was added. 6. The 5 test tubes were observed to see if the precipitate in the test tube dissolved. If the precipitate dissolve this was recorded in the data table as dissolved. Testing with Silver Nitrate 1. Fresh solutions of the compounds were used for this test. 220 drops of the known solutions were added to 4 different 4. 5 drops of Ba(NO3)2 solution were added to each of the 5 test tubes. The contents of each test tube was stirred with a stirring rod and observed for the formation of a precipitate. If a precipitate formed in a test tube, this was recorded in the data table as ppt. If no precipitate was formed in the test tube, this was recorded as no ppt. 5. To only the test tubes in which a precipitate was formed, 10 drops of HNO, solution was added. 6. The 5 test tubes were observed to see if the precipitate in the test tube dissolved. If the precipitate dissolve this was recorded in the data table as dissolved. Testing with Silver Nitrate 1. Fresh solutions of the compounds were used for this test. 2. 20 drops of the known solutions were added to 4 different test tubes. 3. 20 drops of the unknown solution were added to a 5th test tube. 4. 5 drops of AgNO3 were added to each of the 5 test tubes. The contents of each test tube was stirred with a stirring rod. If a precipitate formed in the test tube, this was recorded as ppt. If no precipitate was formed in the test tube, this was recorded as no ppt. 5. To only the test tubes in which a precipitate was formed, 10 drops of HNO, solution was added. 6. The 5 test tubes were observed to see if the precipitate in the test tube dissolved. If the precipitate dissolved this was recorded in the data table as dissolved. The following data was collected for this lab: Data: Substance HNO, Ba(NO), HNO, AgNO, HNO, NaCl No no ppt dissolved no ppt dissolved Na,Co, Yes did not ppt dissolved ppt did not dissolve Na,HPO, No ppt dissolved ppt dissolved Na SO, No did not ppt dissolved ppt dissolved Unknown No ppt dissolved ppt dissolved
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


