Question: i neeeed help plzzz The rate constant for the second-order reaction: 2NOBr(g)2NO(g)+Br2(g) is (Ms)0.80 at 10.0C. starting with a concentration of 0.86M, calculate the concentration
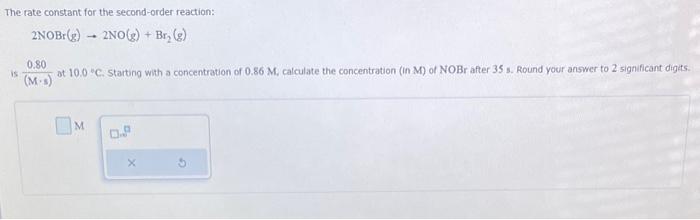
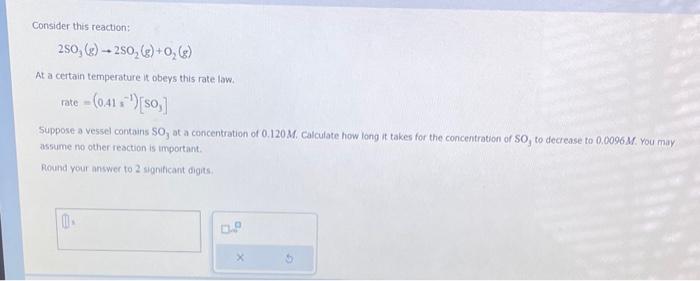
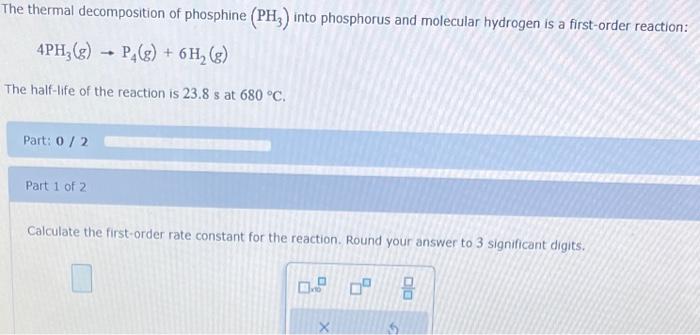
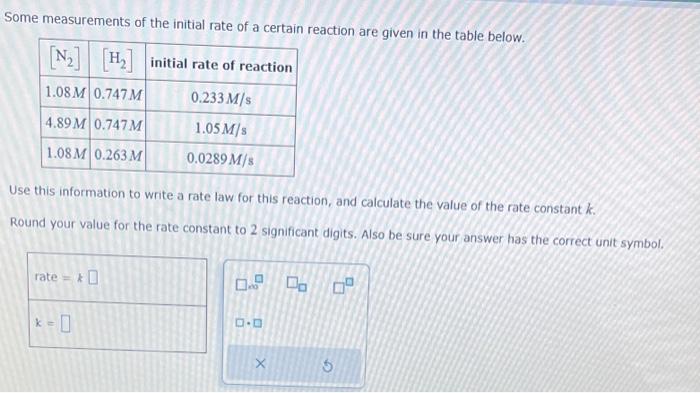
The rate constant for the second-order reaction: 2NOBr(g)2NO(g)+Br2(g) is (Ms)0.80 at 10.0C. starting with a concentration of 0.86M, calculate the concentration (in M ) of NOBr after 35 s. Round your answer to 2 significant digits. M Consider this reaction: 2SO3(g)2SO2(g)+O2(g) At a certain temperature it obeys this rate law. rate=(0.41s1)[SO3] Suppose a vessel contains SO3 at a concentration of 0.120M. Calculate how long it takes for the concentration of : SO3 to decrease to 0.0096M. You may assume no other reaction is important. Round your answer to 2 significant digits: The thermal decomposition of phosphine (PH3) into phosphorus and molecular hydrogen is a first-order reaction: 4PH3(g)P4(g)+6H2(g) The half-life of the reaction is 23.8s at 680C. Part: 0 / 2 Part 1 of 2 Calculate the first-order rate constant for the reaction. Round your answer to 3 significant digits. Some measurements of the initial rate of a certain reaction are given in the table below. Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 2 significant digits. Also be sure your answer has the correct unit symbol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


