Question: I only need the answer to part b.) the answer is 58.1 km/hour please show all work on how to get to that answer Closed

I only need the answer to part b.) the answer is 58.1 km/hour please show all work on how to get to that answer
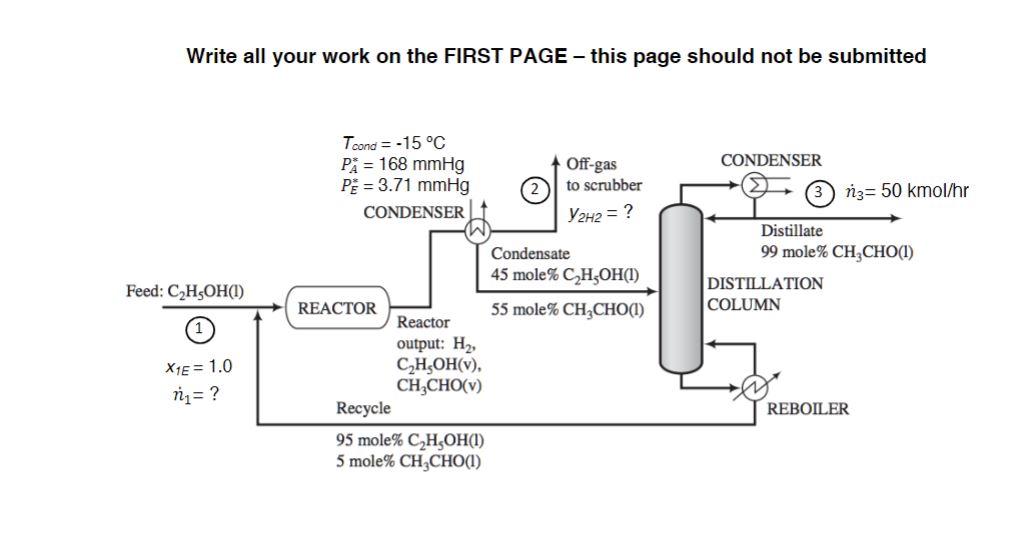
Closed Book/Closed Notes/Open Calculator Acetaldehyde (CH3CHO=A) is synthesized by the dehydrogenation of ethanol (C2H5OH=E) : C2H5OHCH3CHO+H2 Fresh feed (Stream 1) is blended with a recycle stream, and the combined stream is fed to a reactor. Gases leaving the reactor are cooled to 15C condensing the acetaldehyde (PA=168 mmHg) and unreacted ethanol (PE=3.71mmHg). The condensate, which contains 45 mole\% ethanol, is in equilibrium with the off-gas to the scrubber (Stream 2) and is sent to a distillation column that produces a distillate containing 99 mole\% acetaldehyde and a bottoms product that constitutes the recycle blended with fresh feed to the process. The production rate of the distillate (Stream 3) is 50kmol/hr. The pressure throughout the process is 760mmHg absolute. (a) Find the composition of hydrogen in Stream 2,y2H2. (b) Find the flowrate of fresh feed to the process, n1 (kmol/hr). Write all your work on the FIRST PAGE - this page should not be submitted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


