Question: i post the first question but there was no clear answer so please give me the solution of both questions 1. In the reaction; (I2+2S2O32S4O62+2I),12.7g
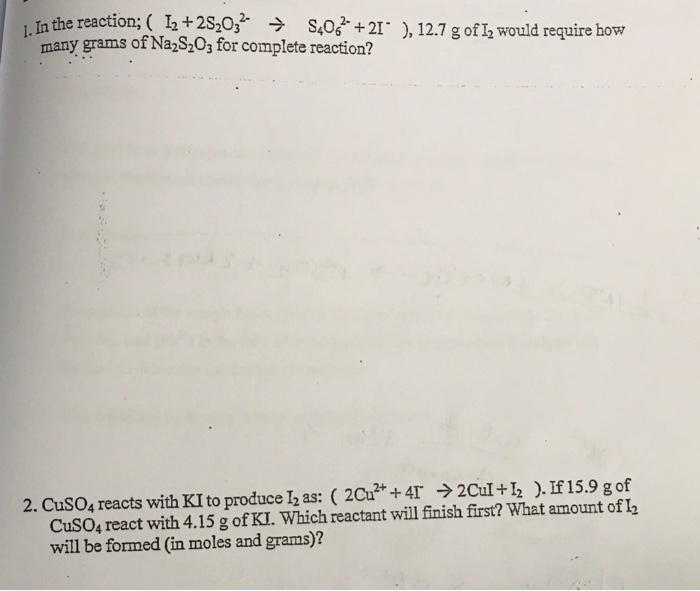
1. In the reaction; (I2+2S2O32S4O62+2I),12.7g of I2 would require how many grams of Na2S2O3 for complete reaction? 2. CuSO4 reacts with KI to produce I2 as: (2Cu2++4I2CuI+I2). If 15.9g of CuSO4 react with 4.15g of KI. Which reactant will finish first? What amount of I2 will be formed (in moles and grams)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


