Question: I. PROBLEM SOLVING. Solve the following problems as neatly and legibly. (50 points each) 1. A gas cylinder with a volume of 2.50m contains 1
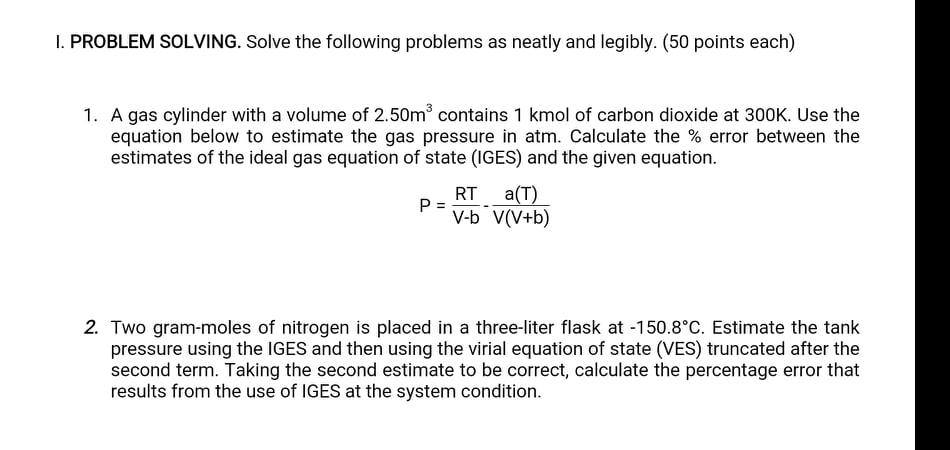
I. PROBLEM SOLVING. Solve the following problems as neatly and legibly. (50 points each) 1. A gas cylinder with a volume of 2.50m contains 1 kmol of carbon dioxide at 300K. Use the equation below to estimate the gas pressure in atm. Calculate the % error between the estimates of the ideal gas equation of state (IGES) and the given equation. RT (T) P = V-b V(V+b) 2. Two gram-moles of nitrogen is placed in a three-liter flask at -150.8C. Estimate the tank pressure using the IGES and then using the virial equation of state (VES) truncated after the second term. Taking the second estimate to be correct, calculate the percentage error that results from the use of IGES at the system condition
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


