Question: I solved this Biophysics problem myself. But obviously there are a lot of places that are wrong. Let the experts see what's right and what's
I solved this Biophysics problem myself.
But obviously there are a lot of places that are wrong.
Let the experts see what's right and what's wrong and explain to me what's the right answer!
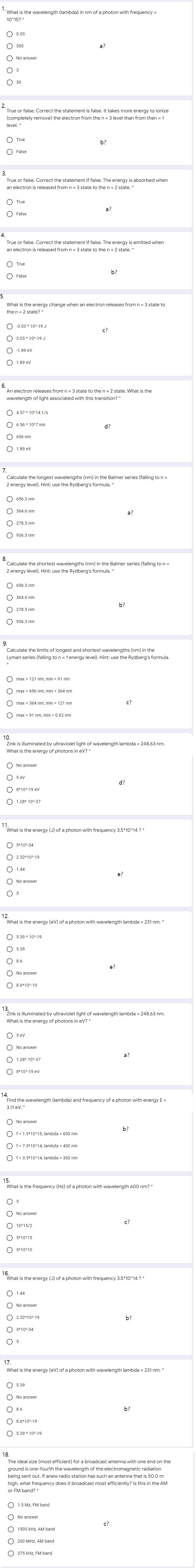
What is the wavelength (lar f a photon with frequency = 10*15? " 30 No answer 0 3 30 True or false. Correct the statement is false. It takes more energy to ionize (completely remove) the electron from the n = 3 level than from then = 1 O True False True or false . Correct the statement if false. The energy is absorbed when an electron is released from n = 3 state to the n = 2 state. " True Fal True or false. Correct the statement if false. The energy is emitted when an electron is released from n = 3 state to the n = 2 state." Fals b? what is the energy then = 2 state? * releases from n = 3 state to O -3.03 * 10%-19 J 3.03 * 10%-19 J O -1.89 ev 1.89 ev An electron releases from n = 3 state to the n = 2 state. What is the wavelength of light associated with this transition? * 4.57 * 10414 1/S O 6.56 * 1047 nm 656 nr 1.89 ev Calculate the longest wavelengths (nm) in the Balmer series (falling to n - 2 energy level). Hint: use the Rydberg's formula. " O 656.3 nm 364.6 nm 278.5 nm 936.3 nm "Calculate the shortest wavelengths (nm) in the Balmer series (faling to n = 2 energy level). Hint: use the Rydberg's formula. " 656.3 nm 364.6 nm 278.5 nm 936.3 nm Calculate the limits of longest and shortest wavelengths (nm) in the Lyman series (falling to n = 1 energy level). Hint: use the Rydberg's formula. max = 121 nm, min - 91 nm max - 656 nm, min - 364 nm max = 364 nm, min = 121 nm max = 91 nm, min = 0.62 nm Zink is illuminated by ultraviolet light of wavelength lambda = 248.63 nm. What is the energy of photons in ev? * O No answer O sev 8*104-19 ev 1.28- 10 -37 What is the energy (J) of a photon with frequency 3.5*10-14 ? * O 5+104-34 O 2.32*10%-19 O 14 O No answer 5 What is the energy (ev) of a photon with wavelength lambda = 231 nm. * 5.39 * 104-19 5.3 8.6 No answer 8.6+104-19 Zink is illuminated by ultraviolet light of wavelength lambda = 248.63 nm. What is the energy of photons in ev? * O 5 ev No answer 1.28* 10 -37 a O 8*104-19 ev 3.11 ev. * Find the wavelength (lambda) and frequency of a photon with energy E = No answer O f= 1.3*10*15, lambda = 600 nm 1= 7.5*10*14, lambda = 400 nm Of = 3.5*10*14, lambda = 300 nm What is the frequency (Hz) of a photon with wavelength 600 nm? * O O No answer O 10*15/2 c? O 5+10415 5+10410 What is the energy (J) of a photon with frequency 3.5*10*14 ? * O 1.44 No answer 2.32*10%-19 O 5+104-34 O 3 17. What is the energy (ev) of a photo wavelength lambda - 231 nm. " O 5.39 No answer b ? 8.6*104-19 5.39 * 104-19 18. The ideal size (most efficient) for a broadcast antenna with one end on the ground is one-fourth the wavelength of the electromagnetic radiation being sent out. If anew radio station has such an antenna that is 50.0 m or FM band? " hat frequency does it broadcast most efficiently? Is this in the AM O 1.5 MZ. FM band O No answer 1500 KHZ. AM band c? O 200 MHz. AM band 375 KHz, FM band
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


