Question: I still do not get itbhow to do this question? How to do it to get the answer given?Help please with clear explanation? QUESTION 2

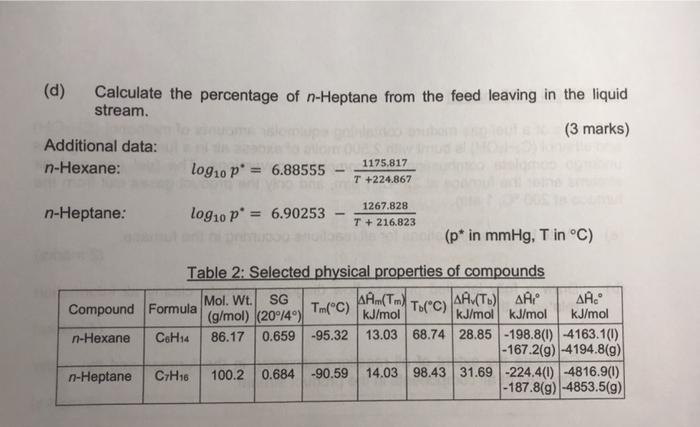

QUESTION 2 (25 marks) 50 kg/h of a liquid feed containing an equimolar mixture of n-Hexane and n-Heptane enters a flash drum which operates at 1.10 atm and 38 C. The liquid and vapour streams leaving the flash drum are in equilibrium. The vapour product stream contains 60 mol% n-Hexane and 40 mol% n-Heptane. (a) Draw a fully labelled process flow diagram. Indicate clearly all information related to the various streams. (4 marks) (b) Determine the operating temperature of the flash drum using Raoult's law and molar composition of the liquid stream leaving the flash drum. (Your answer must be within 11.0 % error.) (12 marks) (c) Calculate the mole ratio of the liquid to vapour flowrate leaving the flash drum. (6 marks) (d) Calculate the percentage of n-Heptane from the feed leaving in the liquid stream. (3 marks) Additional data: n-Hexane: log10 p = 6.88555 1175,817 T +224.867 n-Heptane: log10 p = 6.90253 1267.828 T + 216.823 (p* in mmHg, T in C) Table 2: Selected physical properties of compounds Mol. Wt. SG AA (Tm) AA (T) AA Compound Formula T.(c) AA. (g/mol) (20/4) Tm(C) kJ/mol kJ/mol kJ/mol kJ/mol n-Hexane C6H14 86.17 0.659 -95.32 13.03 68.74 28.85-198.8(0) -4163.1(1) - 167.2(9) -4194.8(9) n-Heptane C7H16 100.2 0.684 -90.59 14.03 98.43 31.69 -224.4(0) -4816.9(0) -187.8(9) -4853.5(9) 2b) 87.0 degC, x(C6) = 0.385, x(c7) = 0.623, 2c) 0.87 2d) 58.0%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


