Question: I think its the first option but that's wrong :( In first ten minutes of the reaction 2O3(g)3O2(g), the amount of ozone (O3) lost is
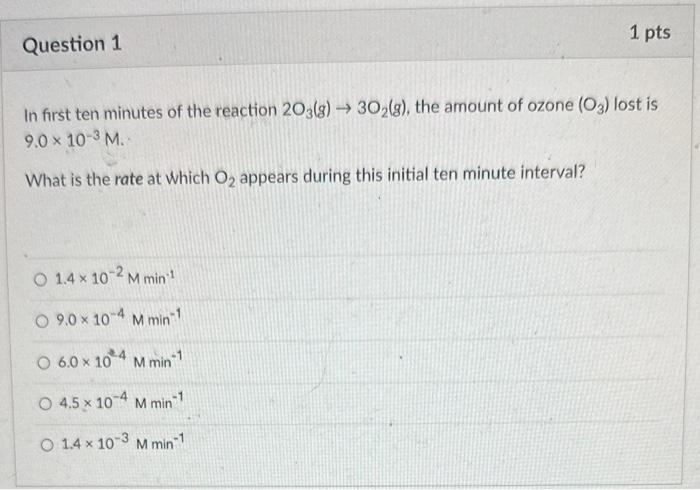
In first ten minutes of the reaction 2O3(g)3O2(g), the amount of ozone (O3) lost is 9.0103M What is the rate at which O2 appears during this initial ten minute interval? 1.4102Mmin19.0104Mmin16.01024Mmin14.5104Mmin11.4103Mmin1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


