Question: I understand what the difference is between cis & trans, but I don't understand how to use that to dissect question #10. Can you break
I understand what the difference is between cis & trans, but I don't understand how to use that to dissect question #10. Can you break it down for me please? Thank you.
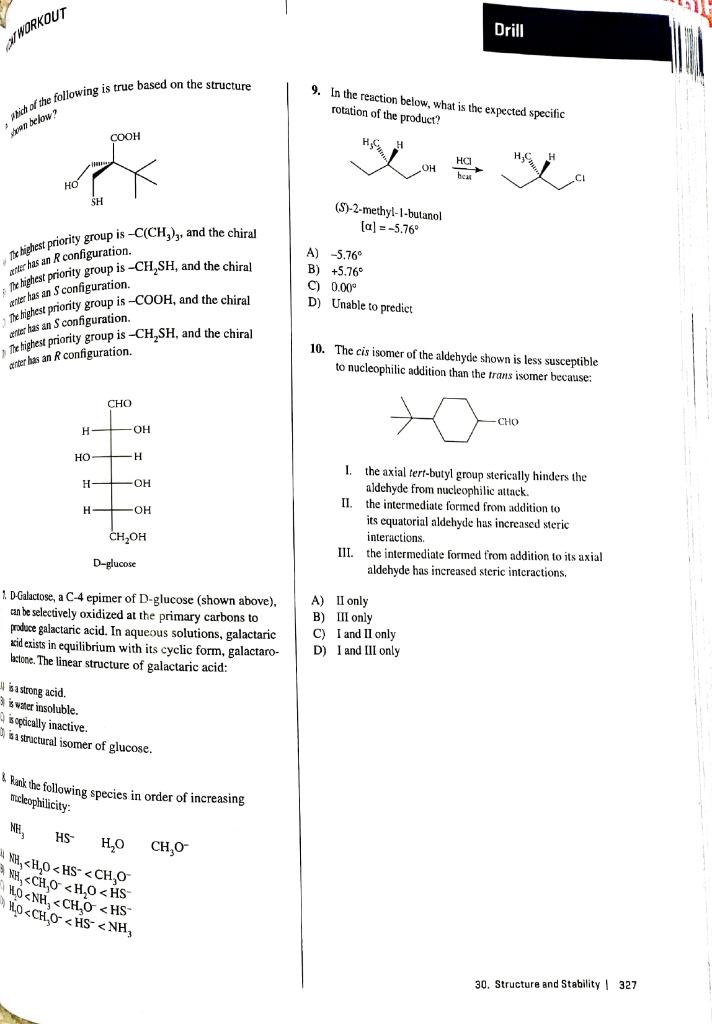
9. In the reaction below, what is the expected specific rotation of the product? (S)-2-methyl-1-butanol []=5.76 antere has an R configuration. Tithightest priority group is CH2SH, and the chiral \$ke thighest priority group is COOH, and the chiral anter has an S configuration. Min tightest priority group is CH2SH, and the chiral at the bas an R configuration. A) 5.76 B) +5.76 C) 0.00 D) Unable to predict I. the axial tert-butyl group sterically hinders the aldehyde from nucleophilic attack. II. the intermediate formed from addition to its equatorial aldehyde has increased steric interactions. III. the intermediate formed from addition to its axial aldehyde has increased steric interactions. 2. D-Galactose, a C-4 epimer of D-glucose (shown above), an be selectively oxidized at the primary carbons to A) II only produce galactaric acid. In aqueous solutions, galactaric B) III only xid exists in equilibrium with its cyclic form, galactaro- C) I and II only batone. The linear structure of galactaric acid: D) I and III only 4 is strong acid. 3 is water insoluble. is opticelly inactive. 7a structural isomer of glucose. 8. Rank the following species in order of increasing micleophilicity: NH3HSH2OCH3O NH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


