Question: I WANT ALL THE ANSWER CORRECTLY! SHOW ALL THE CALCULATION USING HANDWRITING AND DONT CHEAT! PLEASE DONT WAST MY QUESTION! Volumetric analysis of natural gas
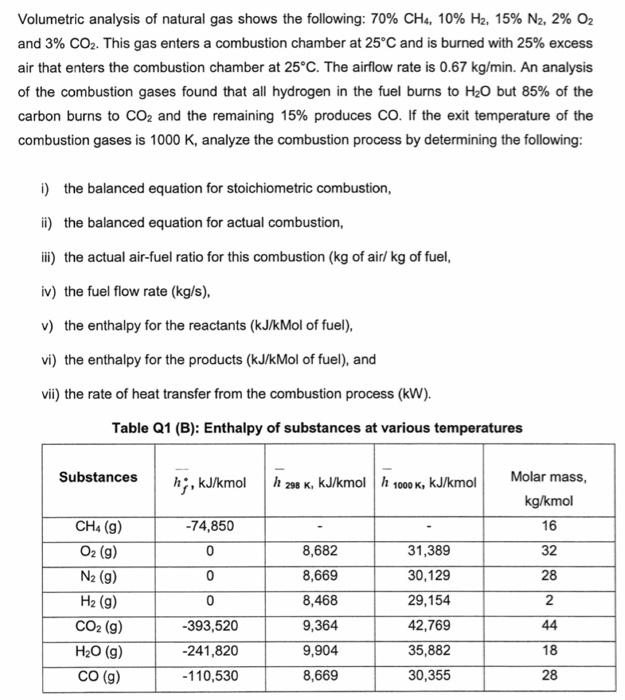
Volumetric analysis of natural gas shows the following: 70%CH4,10%H2,15%N2,2%O2 and 3%CO2. This gas enters a combustion chamber at 25C and is burned with 25% excess air that enters the combustion chamber at 25C. The airflow rate is 0.67kg/min. An analysis of the combustion gases found that all hydrogen in the fuel burns to H2O but 85% of the carbon burns to CO2 and the remaining 15% produces CO. If the exit temperature of the combustion gases is 1000K, analyze the combustion process by determining the following: i) the balanced equation for stoichiometric combustion, ii) the balanced equation for actual combustion, iii) the actual air-fuel ratio for this combustion ( kg of air/ kg of fuel, iv) the fuel flow rate (kg/s), v) the enthalpy for the reactants (kJ/kMol of fuel), vi) the enthalpy for the products ( kJ/kMol of fuel), and vii) the rate of heat transfer from the combustion process (kW)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


