Question: i want an ( excel ) answer as screenshots for ex 4.6 element of chemical reaction engineering Fourth edition... please i don't need anything else
i want an ( excel ) answer as screenshots for ex 4.6 element of chemical reaction engineering Fourth edition... please i don't need anything else beside excel .
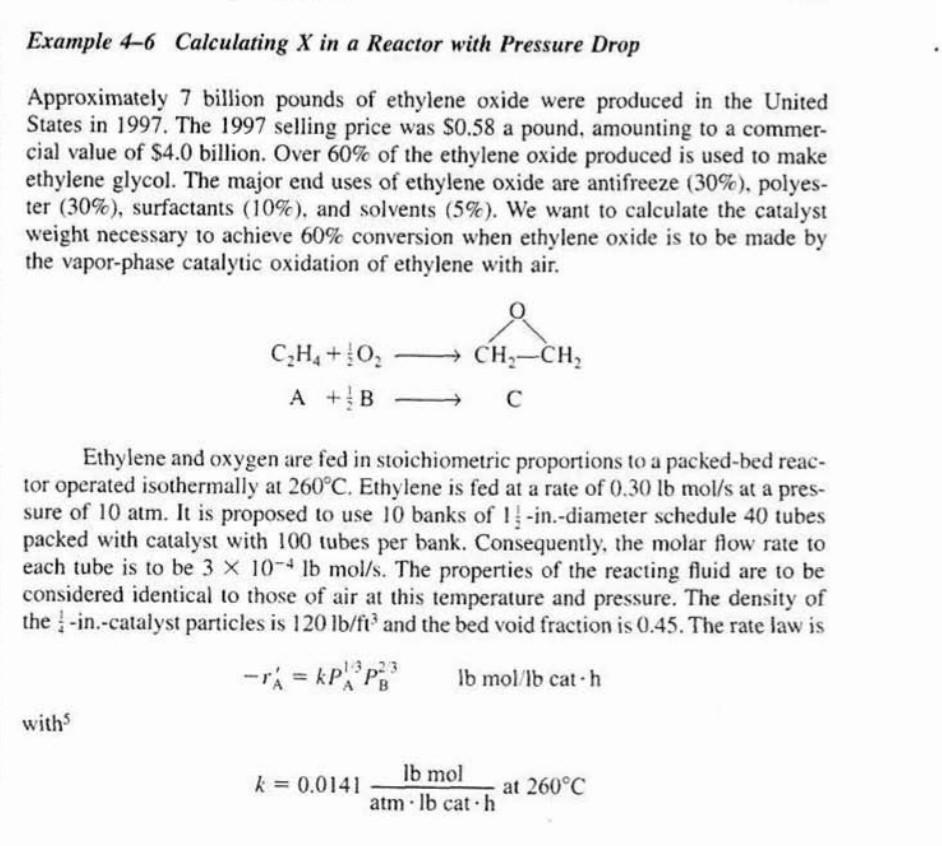
Example 4-6 Calculating X in a Reactor with Pressure Drop Approximately 7 billion pounds of ethylene oxide were produced in the United States in 1997. The 1997 selling price was $0.58 a pound, amounting to a commercial value of $4.0 billion. Over 60% of the ethylene oxide produced is used to make ethylene glycol. The major end uses of ethylene oxide are antifreeze ( 30%), polyester (30%), surfactants (10%), and solvents (5%). We want to calculate the catalyst weight necessary to achieve 60% conversion when ethylene oxide is to be made by the vapor-phase catalytic oxidation of ethylene with air. C2H4+21O2A+21BCH2CH2C Ethylene and oxygen are fed in stoichiometric proportions to a packed-bed reactor operated isothermally at 260C. Ethylene is fed at a rate of 0.30lbmol/s at a pressure of 10atm. It is proposed to use 10 banks of 121-in.-diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 3104lbmol/s. The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the 41-in.-catalyst particles is 120lb/fl3 and the bed void fraction is 0.45 . The rate law is rA=kPA13PB23lbmol/lbcath with 3 k=0.0141atmlbcathlbmolat260C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


